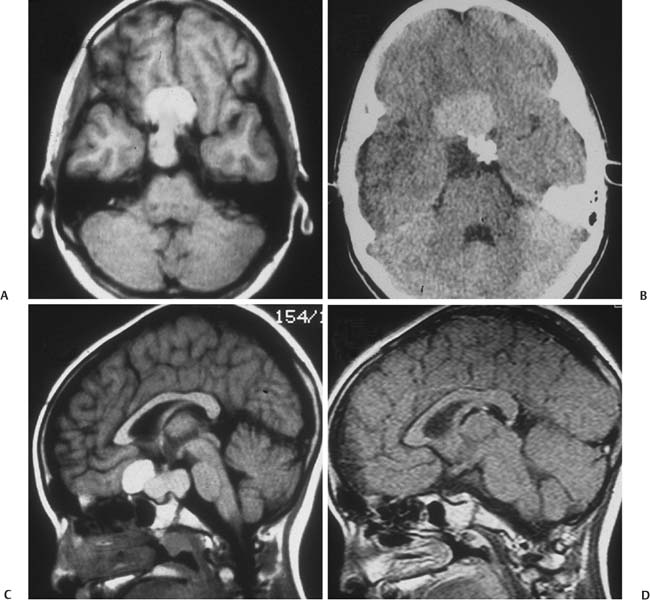
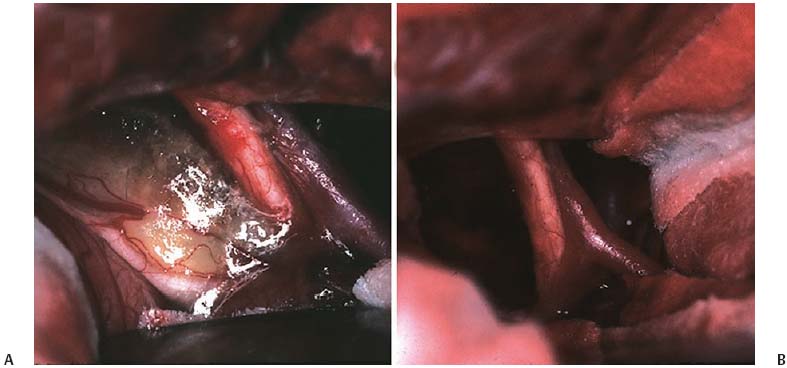
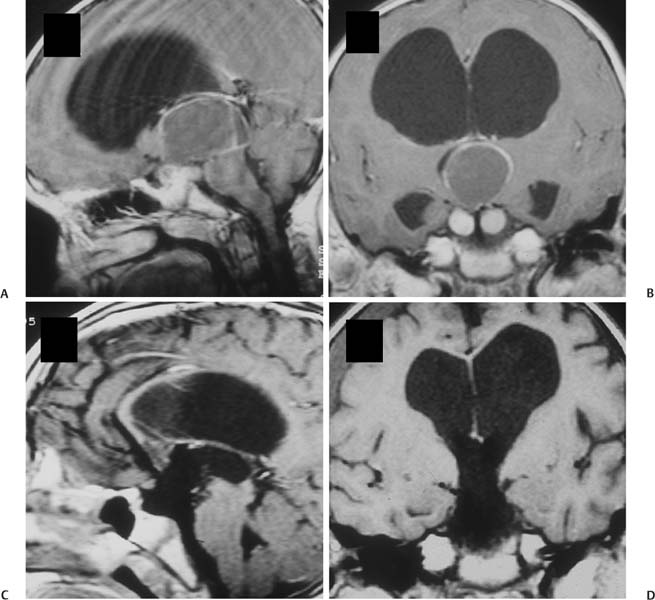
6 Craniopharyngioma Craniopharyngiomas comprise roughly 3% of all intracranial neoplasms1,2 and are the most common nonglial brain tumor of childhood, constituting 6 to 8% of all pediatric brain tumors.3–5 On a population scale, however, they are relatively rare lesions, with an incidence of only 0.13 per 100,000 person years.6 Fewer than 350 combined adult and pediatric craniopharyngiomas are diagnosed each year in the United States, and less than half of these occur in children.6,7 Thought to arise from embryological remnants of the craniopharyngeal duct, these benign epithelial neoplasms with solid, cystic, and calcified components can arise anywhere along an axis from the third ventricle to the pituitary gland.8–12 The benign histology of craniopharyngiomas, however, belies their rather malignant clinical course in children. Described by Harvey Cushing as “one of the most baffling problems to the neurosurgeon,”13 their close proximity to the visual apparatus, circle of Willis, pituitary stalk, and hypothalamus predisposes these patients to severe adverse sequelae both at presentation and following treatment. Common findings include headache, vision loss, diabetes insipidus, panhypopituitarism, short stature, hypothalamic dysfunction with behavioral and memory disturbances, hyperphagia, and obesity. Debate persists regarding the optimal management of craniopharyngiomas. Regardless of selected management strategy, however, definitive tumor control or cure should be the goal of any treatment for pediatric craniopharyngiomas. Two critical factors for potential cure are extent of surgical excision and cranial irradiation. Some centers advocate radical resection for surgical cure, whereas others favor limited resection followed by radiation therapy to limit injury to the hypothalamus. Both major paradigms provide similar rates of disease control and overall survival.14–30 Although radical resection may have a higher potential for immediate perioperative morbidity,14,20,31–36 limited resection and radiation therapy cause more delayed morbidity, including panhypopituitarism, visual deterioration, cognitive and attentional dysfunction, secondary central nervous system neoplasms, and cerebrovasculopathy, namely moyamoya disease.22,37–45 Palliative procedures, such as stereotactic cyst aspiration and Ommaya reservoir drainage, may provide relief from compression of neural and visual structures, but these effects are invariably transient. Progressive solid and cystic tumor recurrence and growth are inevitable. We believe such therapies should not be considered definitive or adequate treatment early in the course of disease. The relative scarcity of craniopharyngiomas, the persistent lack of consensus regarding optimal treatment, and the potential morbidity of all forms of treatment combine to make evaluations of the optimal management strategy difficult, if not impossible. Given similar rates of disease control and survival with the two main treatment strategies, the focus of outcome assessment has shifted to quality of life metrics.22,34,46–50 However, detailed quality of life outcomes from large series of uniformly treated patients are scarce. Here, we describe our preferred treatment paradigm for craniopharyngiomas in children—radical resection with the aim of surgical cure. Depending on the clinical status and age of the patient prior to surgery, we prefer a complete evaluation by various specialists that includes opthalmologic, endocrinologic, and neuropsychological testing. Parents and families are counseled as to the expected short- and long-term postoperative course. Our preoperative imaging protocol consists of magnetic resonance imaging (MRI) with frameless stereotactic image acquisition and computed tomography (CT). CT provides detailed information about the extent and location of tumoral calcification. Careful evaluation of multiplanar MRI is essential to understand the often complex relationship that craniopharyngiomas have to the visual apparatus, hypothalamus, and surrounding vasculature and will lead to improved outcomes. First, the location of the tumor in relation to the optic apparatus must be determined. Tumors can be entirely subchiasmatic primarily within the sella, prechiasmatic with or without subfrontal extension, retrochiasmatic involving the floor of the third ventricle and hypothalamus, or have a complex relationship to the chiasm with both pre- and retrochiasmatic components. Second, attention must be paid to the relationship of the dorsal aspect of the tumor and the hypothalamus. Increased involvement and deformation of the hypothalamus have been shown to predict the level of preoperative hypothalamic dysfunction, as well as the operative morbidity of resection. Third, as craniopharyngiomas enlarge, they can form multilobulated cysts that extend along the pathways of least resistance and invade nearby anatomical spaces in the anterior, middle, and posterior fossae. These extensions must be recognized to optimize the surgical approach and minimize retraction injury to normal brain parenchyma. Given the variability of the precise location and size of craniopharyngiomas, a variety of approaches have been described by different surgeons. These include the sub-frontal,3,5,51–54 pterional,14,17,36,55,56 combined,15,28,30,32,34 bifrontal interhemispheric,57,58 transcallosal,59 subtemporal,60 transpetrosal,61 and transsphenoidal approaches.62–65 We prefer a modified pterional exposure that includes removal of the supraorbital rim, anterior orbital roof, and zygomatic process of the frontal bone. This approach provides the shortest, most direct route to the suprasellar region. It minimizes frontal and temporal lobe retraction with wide splitting of the sylvian fissure, allows early release of cerebrospinal fluid (CSF) from the sylvian and carotid cisterns to aid in brain relaxation, and provides early visualization of the carotid arteries and optic apparatus. Tumors extending from the pontomedullary junction to above the foramen of Monro can be successfully and safely removed using this approach without the need for corticectomy, sacrifice of the olfactory nerve, or potential cognitive dysfunction from retraction of both frontal lobes. Surgical adjuncts include the Cavitron Ultrasonic Surgical Aspirator (CUSA; Tyco Healthcare, Mansfield, Massachusetts), frameless stereotaxy, and rigid and flexible endoscopes and should be used when appropriate. Recently, we have found that endoscopic visualization during dissection of tumor from the ventral surface of the chiasm and floor of the third ventricle greatly enhances the safety of tumor removal in this critical region and allows complete removal of small fragments of tumor and/or calcium deposits that may or may not contain viable tumor cells. The endoscope is also useful for intraventricular visualization and potential resection of tumor that lies within the third or lateral ventricles not accessible via the transsylvian approach. We reserve the transsphenoidal approach for tumors that are primarily or completely within the sella turcica. Following induction and intubation, patient positioning, and stereotaxy registration, dexamethasone (0.1 mg/kg), phenytoin (15 mg/kg), and cephalexin (25 mg/kg) are administered. Mannitol (0.25 g/kg) is than given at the time of skin incision to aid in brain relaxation. The diuretic effect is maximal within 1 hour of infusion and will ideally have its maximal effect at the time of brain and tumor manipulation. Hyperventilation and progressive drainage of CSF from the sylvian and basal cisterns will usually provide excellent brain relaxation, even in the presence of hydrocephalus. Ventricular drainage is reserved for cases refractory to these maneuvers or in cases of severe, increased intracranial pressure unresponsive to medical management. However, if severe hydrocephalus is present or if there is a significant solid tumor component superiorly within the third ventricle, a 4 mm endoscope is placed into the lateral ventricle and held in place with a rigid retractor. This maneuver allows for alternation of visualization and dissection of tumor from the endoscopic, intraventricular, or microscopic transsylvian routes. A Z-plasty skin incision posterior to the hairline is performed from the tragus to just beyond the midline. The temporalis fascia and muscle are sharply incised with a no. 15 blade and bluntly dissected off the underlying calvarium with a periosteal elevator to allow for excellent reapproximation at the end of the case and minimize temporalis muscle atrophy. A one-piece, modified pterional craniotomy with removal of the anterior orbital roof, supraorbital rim, and zygomatic process of the frontal bone is then performed with the craniotome and chisel and mallet. A brain retractor is used to prevent injury to the orbital contents or lacerate the periorbita during the orbital and supraorbital osteotomies. The dura is dissected from the sphenoid bone, which is removed with rongeurs down to the supraorbital fissure. The dura is then elevated with a dural hook and incised in a C-shaped fashion. Especially for large tumors that distort the anatomy of or extend beyond the suprasellar cisterns, identification of the vascular anatomy provides critical internal landmarks for safe navigation. Laterally, the sylvian fissure is widely split, and the branches of the middle cerebral artery are identified. The arachnoidal dissection of the fissure proceeds medially to bifurcation of the internal cerebral artery. Once the carotid artery comes into view, careful inspection of the anterior cerebral artery, optic nerve, chiasm, and/or tracts is performed to understand the relationship of these structures to the tumor (Figs. 6.1 and 6.2). Fig. 6.1 This 9-year-old girl presented with severe, progressive headache. On examination, the child was found to have a partial left cranial nerve III palsy and 20/40 visual acuity on the left. (A,C) Following administration of gadolinium, magnetic resonance imaging (MRI) revealed a 4 cm, mixed cystic and solid tumor with a postfixed chiasm. (B) Solid calcification in the left suprasellar region was demonstrated on computed tomography (CT). (D) Via right pterional craniotomy, she underwent gross total resection (GTR) of the adamantinomatous craniopharyngioma with transient worsening but eventual improvement in her CN III palsy. Her visual acuity improved to 20/25 following surgery. Despite stalk preservation, she developed diabetes insipidus (DI) and requires DDAVP. She is now 18 years following GTR, has been without disease recurrence and completed graduate school after college. We caution against early decompression of the cystic portion of the tumor, as this can result in redundancy of the tumor capsule and the overlying attenuated arachnoid. This loss of turgor can obscure the planes of dissection. The over-arching strategy for craniopharyngioma resection is to develop an arachnoid plane circumferentially around the tumor within the suprasellar cisterns followed by stalk inspection and possible sectioning. The last and most critical step is manipulation and excision of the dorsal portion of the tumor involving the hypothalamus. Fig. 6.2 (A) Intraoperative photograph following splitting of the right sylvian fissure confirming the prechiasmatic nature of the craniopharyngioma seen in Fig. 6.1A. The left optic nerve is elevated by tumor, rotated into view, and exhibits pallor. (B) Following GTR of the tumor, the optic nerves are decompressed, and vasospasm is evident in the right internal carotid artery and the A1 segment of the anterior cerebral artery. Working in the opticocarotid, prechiasmatic, and carotidotentorial triangles, an arachnoidal plane is developed between the tumor capsule and the arteries of the circle of Willis. Careful attention must be paid to ensure the preservation of the basal perforators. This plane is developed in a posterior direction until the basilar artery is identified through the usually intact membrane of Lilliquist. This extracapsular dissection is usually facilitated by well-demarcated and preserved arachnoidal planes. In the case of recurrent tumors, these planes can be heavily scarred and may require increased use of sharp microdissection. Following separation of the cerebral vasculature from the tumor capsule, the cyst can now be aspirated and solid components debulked. All attempts should be made to preserve the capsule of the tumor to allow gentle traction for eventual dissection of the tumor from its remaining attachments, especially the floor of the hypothalamus. Continuing to respect arachnoidal planes, the tumor is then dissected free of the optic chiasm, the contralateral carotid arteries, and its branches. Although an attempt is always made to identify and preserve the pituitary stalk, we have found this successful in only 30% of patients. We recommend sectioning the stalk as distal as possible without compromising negative margins to limit the severity of diabetes insipidus. Following separation of tumor from the entire circle of Willis, pituitary stalk, and optic apparatus, the capsule is grasped, and using a combination of gentle traction and blunt dissection, a gliotic plane is developed between the dorsal aspect of the tumor and the floor of the third ventricle and hypothalamus in the region of the tuber cinereum. Following tumor removal, the entire tumor bed must be inspected for residual disease with either a micromirror or an angled endoscope. Papaverine-soaked Gelfoam pledgets are then placed around the arteries of the circle of Willis to help ameliorate vasospasm (Fig. 6.2) and are removed prior to dural closure. If the tumor has a significant retrochiasmatic or intraventricular component, the lamina terminalis must be fenestrated (Fig. 6.3). The lamina terminalis is distinguished from the chiasm by its pale, avascular appearance and is often dis-tended and attenuated by the underlying tumor. Tumor within the third ventricle can be delivered simultaneously through the lamina terminalis, as well as from below the chiasm. We find the use of a 4 mm endoscope inserted into the third or lateral ventricle to be extremely helpful to assist in the delivery of the intraventricular component of the tumor, obviating the need for a transcallosal approach to achieve complete resection. For tumors with significant extension into the sella turcica, removal of the tuberculum sellae and posterior planum sphenoidale may be necessary to gain adequate exposure of the intrasellar space. Following tumor removal, all bony defects into the sinuses must be repaired to prevent postoperative CSF fistulas. Following surgery and neurologic examination, all children are transferred immediately to the pediatric intensive care unit. A multidisciplinary team of pediatric endocrinologists, neuro-oncologists, and intensivists collaborate in the postoperative care. Frequent urine and electrolyte analyses are performed to monitor for and aggressively treat electrolyte disturbances, namely diabetes insipidus. Dexamethasone is tapered over the course of 1 week, and Dilantin is continued for 3 weeks following surgery. Dilantin is continued for extended periods only if seizures occur that are not attributable to electrolyte disturbances. Fig. 6.3 (A,B) This 7-year-old boy presented with headache and behavioral outbursts. MRI revealed a 5 cm retrochiasmatic tumor with significant extension into the third ventricle, causing obstructive hydrocephalus. (C,D) Following GTR of his adamantinomatous craniopharyngioma via a right pterional approach and fenestration of the lamina terminalis, he remained neurologically, visually, and hormonally intact, and his hydrocephalus resolved following tumor removal. He did, however, experience slight worsening of his short-term memory but was able to do well in school and currently attends college. He remains disease-free 14 years following resection. Postoperative MRI and CT are performed within 48 hours following surgery to ensure complete resection. Surveillance MRI and clinical follow-up occur every 3 months during the first year, every 4 months during the second year, every 6 months for the next 3 years, and every year for the next 5 years. Frequent imaging allows for early detection of recurrence while tumors are small and preferably asymptomatic. However, long-term imaging and follow-up are important, as late recurrences have been reported. Regular evaluations by dedicated pediatric endocrinologists, ophthalmologists, and neuro-oncologists are essential in managing these children long term. In the MRI era, radiographically confirmed complete resection is possible in 80 to 100% of patients. Perioperative mortality following aggressive surgery has also declined substantially over the past 2 decades secondary to advances in neuroimaging and microsurgical techniques from over 10% down to 0 to 4% in most current series.3,14–17,20,21,23,30,31,34,36,53,55,56,66–72 Multiple authors have reported surgeon experience with craniopharyngiomas has a significant impact on the likelihood of achieving complete resection and good functional outcomes.26,34 Surgeons performing more than two operations per year for radical resection had good outcomes in 87% of children compared with only 52% in those performing fewer.26 Numerous centers have reported excellent rates of disease control and functional outcomes with the strategy of radical resection. In a large series by Zucarro,30 complete resection was achieved in 69% of 153 children. All children who underwent complete resection were in school and no more than 1 year behind in grade level, in contrast to only 62% of children who had limited resection and radiation. Di Rocco et al16 reported complete resection in 78% of 54 children treated with curative surgical intent. Overall improvement in intelligence quotient (IQ) occurred following resection in their series with a mean postoperative IQ of 112 (range 95–130). All but 2 of 50 surviving patients enjoy normal social interactions. In a series by Hoffman et al,53 26 of 27 children who underwent aggressive resection had IQ scores at or above average levels. Although 16 children had memory deficits, 14 of them attended regular schools. The authors contended that “memory impairment did not interfere with school progress if intelligence was adequate.” Yasargil et al36 reported good outcomes in 72.5% of children after initial surgery, and Fahlbusch et al55 reported functional independence in 78% of adult and pediatric patients following radical resection. In our series of 86 children who underwent radical resection of craniopharyngiomas, gross total resection (GTR) was accomplished in all 57 (100%) of primary tumors and in 18 of 29 (62%) of recurrent tumors with acceptably low morbidity (Table 6.1). In contrast to the findings of other centers of increased morbidity, mortality, and worse functional outcomes at reoperation,14,20,21,36,55,73–77 we found no such differences in our series. Good and excellent functional outcomes were achieved in 80% of children, and over 60% of college-aged patients either attended or graduated from college—a clear indication of the high functionality of the majority of these children. New hypothalamic morbidity occurred in 25% of children and was mild or moderate in all but one case. Fewer than 20% of our patients developed obesity, and only two patients developed severe or morbid obesity. These results contrast greatly with those from a German multicenter study that reported severe obesity in 44% of 185 children treated for craniopharyngiomas using various treatment modalities.48 Although some centers contend that increasing tumor size limits the extent of resection and local disease control,29,31,55,78–84 we agree with other authors30,51,56,85 that size has no impact on the ability to achieve GTR—at least for virgin tumors. Nevertheless, given the large size and multicompartmental nature of giant craniopharyngiomas, a flexible and at times staged approach may be required for successful and safe extirpation of these tumors (Fig. 6.4).
Radical Resection
Treatment Philosophy
Preoperative Evaluation
Surgical Approaches
Operative Technique
Postoperative Care
Outcomes and Complications
| No. of Patients (%) | |
| Primary | Recurrent |
Perioperative mortality | 2 (3.5%) | 1 (3.4%) |
Neurologic morbidity |
|
|
Stroke | 2 (4.0%) | 2 (9.0%) |
Mild hemiparesis | 1 (2.0%) | 0 (0%) |
Transient CN palsy | 8 (16.0%) | 1 (4.0%) |
Permanent CN palsy | 1 (2.0%) | 1 (4.0%) |
Lethargy/abulia | 2 (4.0%) | 1 (4.0%) |
Visual acuity |
|
|
Preoperative deficit | 14 (27.0%) | 15 (60.0%) |
Improved | 10 (19.0%) | 3 (12.0%) |
Stable | 35 (67.0%) | 17 (68.0%) |
Worse | 7 (13.0%) | 5 (20.0%) |
Visual fields |
|
|
Preoperative deficit | 23 (43.0%) | 22 (85.0%) |
Improved | 13 (25.5%) | 7 (27.0%) |
Stable | 25 (49.0%) | 12 (46.0%) |
Worse | 13 (25.5%) | 7 (27.0%) |
Diabetes insipidus |
|
|
Preoperative | 6 (12.0%) | 19 (73.0%) |
Postoperative, new | 33 (73.0%) | 6 (67.0%) |
Postoperative, total | 39 (78.0%) | 25 (89.0%) |
Anterior pituitary dysfunction |
|
|
Mean number hormones required ± SD | 2.5 ± 1.1 | 2.1 ± 0.9 |
Note: There were no significant differences in operative mortality, neurologic, visual, or endocrinologic morbidity rates between patients with primary and recurrent tumors (p>.05).
CN, central nerve; SD, standard deviation.
Although our data did corroborate prior studies reporting worse overall survival rates for children with recurrent tumors,25,29,36,55,73,86
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree