Chapter 102 Snoring
Historical Perspective
Before the 1970s, snoring was thought to be simply an acoustic nuisance. However, with the recognition of obstructive sleep apnea syndrome, snoring began to assume a new importance as the cardinal symptom of this disorder. Investigators began to understand snoring and obstructive sleep apnea as a continuum of sleep-disordered breathing1 and question whether this acoustic nuisance in fact had serious health consequences. As polysomnography became more commonplace, objective evaluation of snoring evolved from a research oddity to an accessible, real-world tool. The 1990s led to the recognition that some nonapneic snorers have frequent respiratory arousals and sleep fragmentation, and the term upper airways resistance syndrome (UARS) was coined to describe these patients (see Chapter 105).
Definitions
The International Classification of Sleep Disorders defines snoring as a “respiratory sound generated in the upper airway during sleep that typically occurs during inspiration but may also occur in expiration.”2 The manual also lists a number of alternate names for nonapneic snoring, including “primary snoring,” “simple snoring,” “benign snoring,” “habitual snoring,” and “heavy snoring.”
Pathophysiology and Measurement of Snoring
Snoring is a sound produced by the vibrating structures of the upper airway. It is not caused by a single, localized abnormality within the airway. Endoscopic and imaging observations of the upper airway in snorers, during natural or induced sleep, demonstrate that any membranous part of the upper airway that lacks cartilaginous support can vibrate.3 This includes the soft palate, uvula, faucial pillars, pharyngeal walls, and the rest of the upper airway, almost to the level of the vocal cords. This diffuse involvement of the upper airway has made successful treatment of snoring more difficult, as many treatments address only a particular airway segment.
Several theoretical models of the human upper airway have been developed (see Chapter 101).4–6 These models include the effects of airway wall compliance, gas density, and airway dimensions on the pressure–flow relationships in the upper airway. They predict that depending on the mechanical properties of the pharynx and the density of gas flowing through it, the walls of the upper airway can be stable (normal breathing), can vibrate or flutter (snoring), or can narrow or collapse completely (obstructive hypopnea or apnea, respectively). Some predictions have been verified experimentally. For example, alcohol ingestion promotes snoring by reducing pharyngeal dilator muscle tone and increasing airway wall compliance, in effect making the pharynx more floppy. On the other hand, breathing a lower-density, helium-containing gas mixture can improve snoring by promoting laminar over turbulent airflow, favorably altering the pressure–flow relationship in the pharynx and thereby reducing vibratory sounds.
Methods of measuring snoring vary,7,8 but they generally include one or several of the following parameters: maximum sound intensity, percentage of sleep time spent above a threshold sound level, and number of sound intensity spikes above threshold level. However, these measurements are very specific to an individual laboratory’s configuration and are not standardized. In many laboratories, “measurement” of snoring simply consists of a sleep technologist’s subjective comment. It is therefore not surprising that many patients, particularly those referred only because of their snoring, leave the physician’s office rather disappointed: when they ask about their snoring, all they are told is that they do not have sleep apnea.
Epidemiology of Snoring
Snoring is common, but estimates of its prevalence are imprecise and vary widely among studies. The prevalence of snoring ranges from 5% in men and 2% in women9 to 86% in men and 57% in women.10 Although ethnic differences in snoring prevalence undoubtedly exist, most of the variability is very likely a reflection of differences in data-collecting methodology. Snorers rely on a bed partner or family member to describe their snoring. Such reports are inherently subjective and inconsistent. Furthermore, some studies do not even employ bed-partner questionnaires, using only the subject’s impression of his or her own snoring to determine the prevalence of the condition. Night-to-night variability further compounds the difficulty in interpreting reports of snoring. Not surprisingly, therefore, there is commonly a discrepancy between patient reports of snoring and objective snoring recordings. In one study, 15% of self-confessed snorers did not snore at all when studied in the sleep laboratory. On the other hand, 15% of those who reported never snoring actually snored for at least 50% of the night.
The male predominance observed in all prevalence studies of snoring remains unexplained. Possible contributing factors include differences in the perception and reporting of snoring between male and female bed partners,11 hormonal factors influencing upper airway tone, and differences in pharyngeal anatomy and function. The apparent reduction in snoring with age, observed in some studies, may be related to the loss of a bed partner, altered perception of snoring, survival bias, or other methodologic problems.12 A few special conditions appear to be particularly associated with snoring, such as pregnancy,13,14 presence of nasal polyps,15 or nasal obstruction from other causes.16
It is clinically observed that snorers very often have a history of at least one other affected family member. Hence, genetic factors (see Chapter 103) can influence the prevalence of snoring, perhaps through obesity or through inherited oropharyngeal anatomy.
Health Effects of Nonapneic Snoring
Snoring and Daytime Sleepiness
Some of the evidence for a relationship between snoring and daytime sleepiness comes from the Sleep Heart Health Study, a cross-sectional study of 5777 community-dwelling adults. Participants underwent full polysomnography at home. The authors showed a correlation between daytime sleepiness, as measured by the Epworth Sleepiness Scale (ESS), and self-reported snoring; the correlation was independent of the Respiratory Disturbance Index (RDI). The ESS increased progressively (reflecting increasing degrees of perceived sleep propensity during daily activities) with increasing snoring severity across the study population, from 6.4 in nonsnorers to 9.3 in the heaviest snorers.17 A more recent study also described a correlation between objectively quantified snoring and ESS.18 However, the overall correlation was quite weak, as snoring intensity and AHI together only explained 15% of the variance in ESS. Furthermore, the correlation was only present in patients with an AHI of more than 15 events/hour. This suggests that snoring is not a major cause of sleepiness in patients with mild OSA or nonapneic snoring.
Snoring and Cardiovascular Disease
Many authors have postulated that there is a relationship between snoring and hypertension. In 1975, Lugaresi and colleagues demonstrated that episodes of snoring were associated with acute, simultaneous increases in blood pressure.19 Many epidemiologic, questionnaire-based studies since then have demonstrated an association between snoring and hypertension. However, without available polysomnographic data it is unclear whether the hypertension is associated with nonapneic snoring, obstructive sleep apnea, or both. Several attempts have been made to clarify this issue. In support of the association between snoring and hypertension is the study of Bixler and colleagues,20 who performed polysomnography in 1741 community dwellers and reported that when compared to nonsnorers, nonapneic snorers had an odds ratio of 1.6 (confidence interval [CI], 1.09 to 2.20) for having hypertension (defined either as taking antihypertensive medication or having elevated blood pressure measured at the time of polysomnography). On the other hand, Stradling and colleagues examined a cohort of snorers from general practice who were unlikely to have significant sleep apnea because they had fewer than two episodes of 4% drops in oxygen saturation per hour of sleep; they found no relationship between snoring and hypertension.21 From the available evidence, it would appear that the association between nonapneic snoring and hypertension is, if anything, modest.
Similarly, although epidemiologic studies have demonstrated an association between snoring and cardiovascular disease and stroke, none have convincingly demonstrated that snorers are at risk in the absence of apnea. Marin and colleagues compared long-term cardiovascular outcomes in nonapneic snorers (apnea–hypopnea index [AHI] less than 5 events per hour of sleep), subjects with obstructive sleep apnea, and population-based healthy controls matched for age and body mass index.22 They found that among the nonapneic snorers, there was no increased risk for cardiovascular death and no increase in the rates of nonfatal cardiovascular events. On the other hand, there is intriguing new evidence that snoring may be associated with carotid artery atherosclerosis. In a study of 110 subjects, mainly community volunteers who had nonapneic snoring or mild nonhypoxic obstructive sleep apnea (median AHI 11 events per hour; median 3% oxygen desaturation index 1.5 events per hour, 0% of total sleep time spent with oxygen saturation lower than 91%), the prevalence of carotid atherosclerosis on Doppler ultrasound increased significantly with snoring severity, independent of the AHI.23 This relationship was not seen in the femoral artery, and the authors postulate that transmitted vibrations to the carotid artery might in fact lead to endothelial damage and atherogenesis.
Clinical Features and Diagnostic Assessment
Clinical History
Does the patient in fact have obstructive sleep apnea? Much of the clinical assessment of the snorer is directed toward determining the probability of obstructive sleep apnea or UARS. This is discussed in detail in Chapter 105. The clinician should enquire about nighttime symptoms of obstructive sleep apnea such as frequent awakenings, restless sleep, witnessed pauses in breathing, choking and gasping spells, diaphoresis, and nocturia. Daytime symptoms must also be assessed. Patients with obstructive sleep apnea can experience excessive daytime sleepiness, neurocognitive symptoms such as poor concentration and memory, and mood symptoms such as irritability and depression. Daytime sleepiness can be quantified using a standardized tool such as the Epworth Sleepiness Scale (see Chapter 143). Finally, the history should document medical conditions that may be associated with obstructive sleep apnea, such as hypertension and cardiovascular disease.
Clinical and Laboratory Investigations
Upper Airway Assessment
The anatomic structures of the upper airway can be visualized using fiberoptic nasopharyngoscopy, which is usually carried out by an otolaryngologist. Airway pressure measurements, simulated snoring, and Müller’s maneuver may be performed during endoscopic examination, but there is actually little evidence that such maneuvers can accurately determine the site of obstruction or predict surgical success. In fact, even when nasopharyngoscopy is done during sleep, the findings are a poor predictor of success in palatal surgery for nonapneic snoring.24
Polysomnography
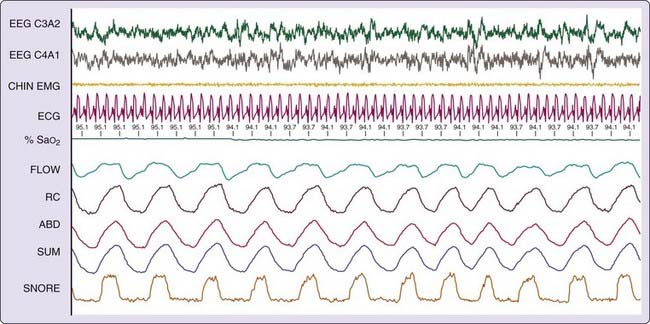
Figure 102-1 demonstrates some polysomnographic findings of nonapneic snoring. Snoring was measured in this instance by a snoring microphone, positioned above the patient’s head. Note the snoring in the bottom channel, in the absence of a reduction in flow, oxygen desaturation, or arousal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree