SEIZURE SEMIOLOGY DURING VIDEO–EEG IN INFANTS AND CHILDREN
Clinical features of focal seizures may differ in pediatric and adult surgical candidates. Independent studies (26–29) of videotaped seizures from patients at separate institutions indicated that the classification of epileptic seizures of the International League Against Epilepsy (29), originally reflecting experience in older patients, was not applicable to infants younger than 3 years of age. In one study (26), only 3 of 21 infants had unmistakable characteristics of localized seizure onset, including clonic jerking of one extremity. In the remaining patients, seizures consisted of a decrease in motor activity with indeterminate level of consciousness and minimal or no automatisms, arising from temporal or temporoparietal regions, or bilateral tonic stiffening sometimes preceded by bilateral eyelid blinking, arising from frontal or frontoparietal regions. In another study of 77 children with temporal lobe epilepsy examining the relationships between etiology, age at onset, and electroclinical findings, auras were typically clear after the age of 6 years, and initial ictal symptomatology consisted of staring with behavior arrest, lip cyanosis, and bland or subtle oral automatisms again reiterating the lack of clear lateralizing or localizing semiology (30). Other authors (27,31) have also noted bilateral motor phenomena during partial seizures in infants. The mechanism is unknown but may include ictal activation of subcortical regions or of the supplementary sensorimotor area. A localized EEG seizure pattern clarifies the focal nature of the epileptogenic process.
Seizure characteristics signaling localized onset in older patients may be absent or unidentifiable in infants. For example, an aura is an important clue to focal onset in older children and adults, but sensory phenomena are difficult to detect and are rarely observed during video–EEG studies in infants (26). Clinical seizure onset may be difficult to notice, especially in mentally impaired young children, and this may create a challenge during diagnostic evaluation like video–EEG and ictal single photon emission computer tomography (SPECT) (32,33). Complex gestural automatisms and altered awareness are hallmarks of many partial seizures in older patients, but assessment of the ictal level of consciousness in infants is fraught with problems, and automatisms, when present, tend to be simple, bland, and predominantly oral. In infants, distinguishing automatisms from normal background behavioral activity can be difficult (26,27).
SCALP EEG PATTERNS, INFANTILE SPASMS, AND FOCAL CORTICAL LESIONS
Within the first 2 years of life, focal cortical lesions may manifest as infantile spasms and hypsarrhythmia (12,34–36). The spasms may be intermixed with partial seizures (Fig. 89.1) or may replace a previous partial seizure type altogether, becoming the only active seizure type (Fig. 89.2). The mechanism is unknown, but a clue may be the relationship between age of onset of spasms and location of the lesion. Koo and Hwang (37) found that spasms began earliest in patients with occipital lesions (mean age, 3 months), appeared later in patients with centrotemporoparietal lesions (mean age, 6 months), and occurred latest in patients with frontal lesions (mean age, 10 months). This timing coincides with maturation in those regions, rapid increases in synaptic density, and sequential myelination that proceed from the back to the front of the brain. Infantile spasms appear to result from an age-related pathologic interaction between a focal cortical lesion and normal developmental processes.
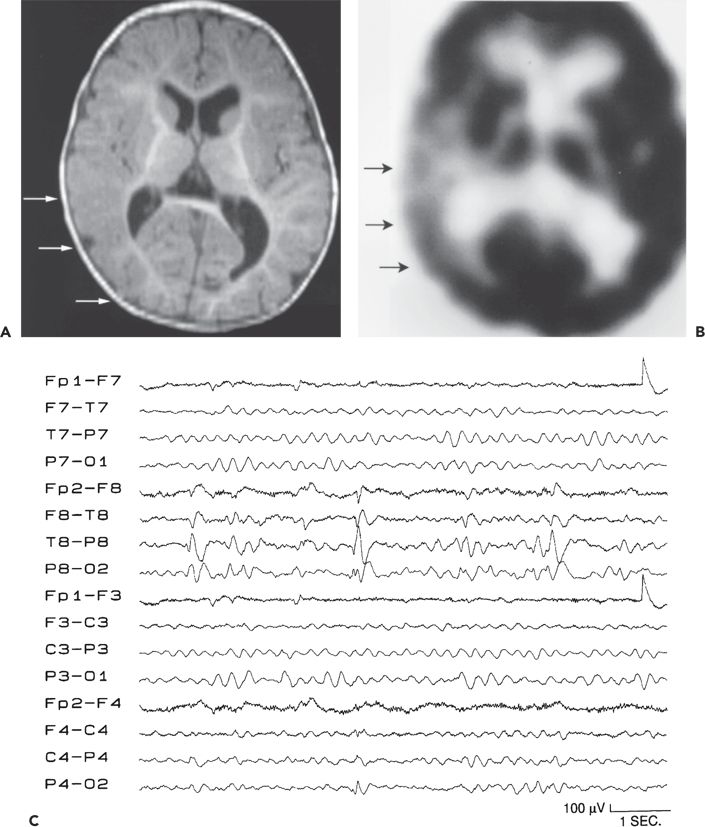
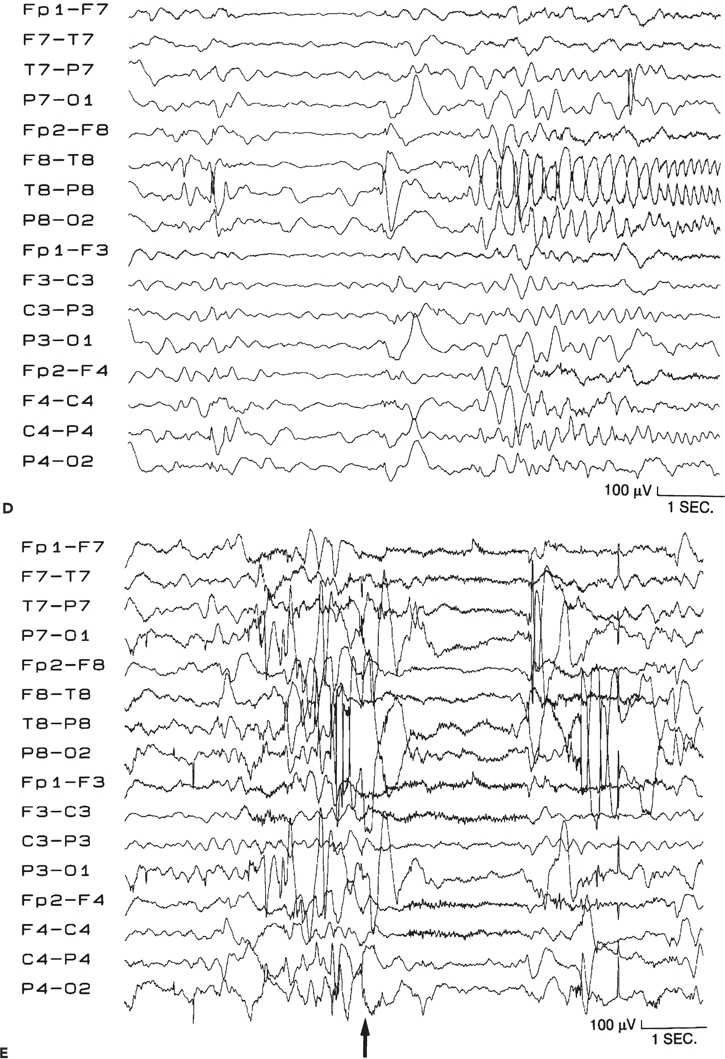
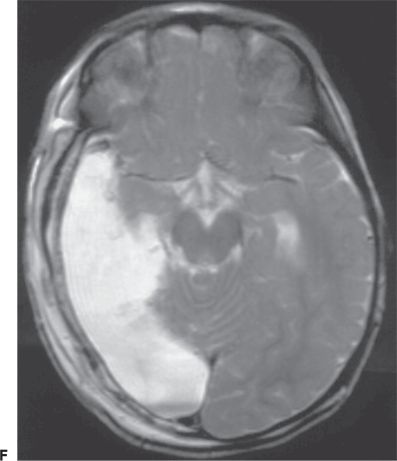
Figure 89.1. Case 1. (All images are of the same patient.) A: Axial magnetic resonance image from an 8-month-old boy, showing focal malformation of cortical development in the right temporo-occipital region (arrows). Findings were subtle and included decreased arborization of the white matter and thickened, poorly sulcated cortex. Seizures began 14 hours after an unremarkable term birth and occurred 20 to 30 times per day. The boy was otherwise normal except for developmental delay. B: 2-[18F]fluoro-2-deoxy- d-glucose PET scan at age 8 months, showing glucose hypometabolism in the right temporo-occipital region (arrows). C: Interictal electroencephalogram at age 8 months, showing right posterotemporal sharp waves (maximum at the T8 and P8 electrodes), slowing, and decreased background activity. D: Ictal electroencephalogram at age 8 months with seizure pattern maximum in the right posterior temporal region (T8 electrode). Seizures involved bilateral clonic eyelid blinking, rhythmic interruption of crying, and bilateral clonic arm twitching. E: Ictal electroencephalogram at age 8 months, showing diffuse electrodecrement (arrow, preceded and followed by movement artifact) during an asymmetric spasm with extension and elevation of both arms (left more than right) and tonic closure of the left eyelid. F: Magnetic resonance image showing the right temporo-occipital resection performed at age 22 months. Fourteen months later, the child still has developmental delay but remains free of seizures off all antiepileptic medication. (A and C–F are from Wyllie E. Surgical treatment of epilepsy in infants and children. Can J Neurol Sci. 2000;27:106–110, with permission.)
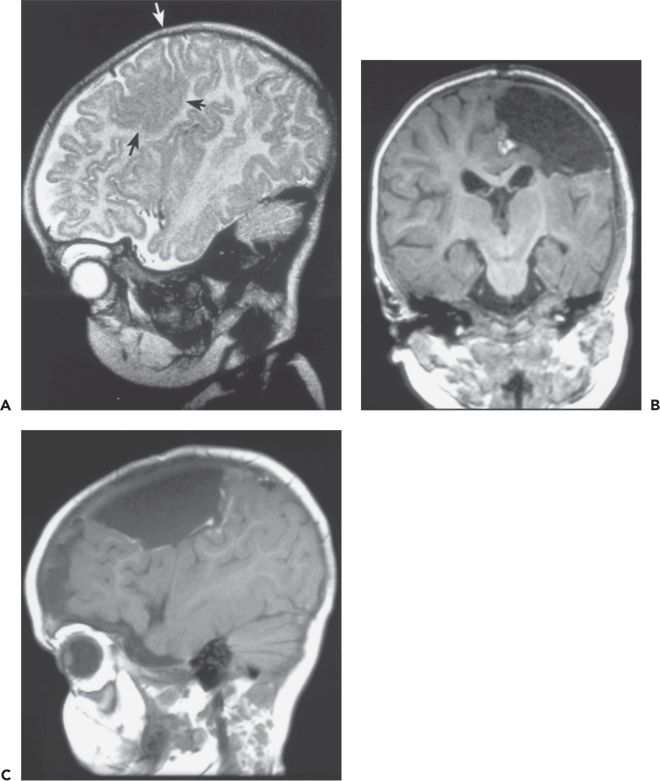
Figure 89.2. Case 2. A: Sagittal magnetic resonance image showing focal malformation of cortical development cerebral dysgenesis (black arrows) in the left posterior frontal lobe extending across the central sulcus (white arrow) into the anterior portion of the postcentral gyrus. The boy was 4 months old at the time of the MRI, with intractable daily seizures since the first day of life after an uncomplicated full-term delivery. Seizures involved clonic jerking of the right arm and leg, with eye deviation toward the left, or opisthotonic posturing with stiffening and extension of all extremities. Ictal and interictal epileptiform discharges were localized to the left central region. Moderately severe right hemiparesis and mild developmental delay were also present. B: Coronal and (C) sagittal scans performed 2 days after cortical resection at age 9 months. Prior to resection, electroencephalographic seizure was recorded over the lesion with intraoperative electrocorticography, and primary hand motor cortex was identified in the same area by intraoperative cortical stimulation. Postoperatively, the hemiparesis was transiently minimally worse, returning to preoperative baseline within days. Twenty-two months later, the child is making developmental progress and has had no seizures on a reduced dose of antiepileptic medication. (A from Wyllie E. Surgical treatment of epilepsy in children. Pediatr Neurol. 1998;19:179–188, with permission.)
Chugani et al. (13,38,39) first emphasized the role of positron emission tomography (PET) and magnetic resonance imaging (MRI) in identifying focal cortical lesions in children with infantile spasms and hypsarrhythmia, describing several patients with cessation or dramatic reduction of seizures after cortical resection or hemispherectomy. Their experience has been replicated elsewhere (2,35,40). Sixty-five percent of affected children are free of seizures after surgery (12), and infantile spasms are not predictive of poor outcome. However, the identification of appropriate surgical candidates may be complicated by the absence of focal EEG seizure patterns in the setting of spasms with diffuse electrodecrements (17,40).
The goal of the presurgical evaluation in patients with infantile spasms is to identify a region of cortical abnormality. The most common finding for surgical planning in this setting is a unilateral lobar, multilobar, or a hemispheric epileptogenic lesion on MRI or PET, usually a malformation of cortical development or encephalomalacia following perinatal cerebral infarction or ischemia. Helpful EEG findings may include a predominance of interictal sharp waves over one region; localized slowing, decreased background activity, or absent sleep spindles over the affected region or hemisphere; unilateral electrodecremental events; asymmetric EEG seizures; or a history of partial seizures (3). Neurologic examination may show evidence of unilateral hemispheric dysfunction with decreased spontaneous movement of one arm (hemiparesis) or gaze preference to one side (homonymous hemianopia).
Generalized epileptiform discharges on scalp EEG in the presence of a congenital or early-acquired focal lesion are not limited to infants. Recently, two reports from Cleveland Clinic described older children and adolescents with a unilateral or strongly asymmetric focal or hemispheric epileptogenic lesion who presented with generalized interictal abnormalities and ictal scalp EEG patterns (41,42). Initially, many of these children were rejected for surgical treatment owing to the presence of generalized EEG findings and lack of localizing EEG data. Because of a high burden of seizures, failure of most treatment modalities, and minimal risk of new postoperative side effects, surgical treatment was generally offered as a last resort with resection of the brain MRI lesion or hemispherectomy in each case. Seizure-free outcome was obtained for 70% of these children with generalized or contralateral EEG abnormalities, and these results were similar to those in a comparison group of children with similar MRI lesions and localized EEG findings. On further analysis of the group with generalized video–EEG abnormalities, the rate of seizure freedom after resection of the lesion was invariable regardless of the presence or absence of focal ictal semiology, generalized slow spike-and-wave complexes, and proportion (30% to 100%) of the generalized or contralateral ictal and interictal epileptiform discharges (42). Postoperative EEG in seizure-free children typically showed resolution of the generalized or contralateral epileptiform discharges, which were often nearly continuous on preoperative EEG, especially during sleep (Fig. 89.3).
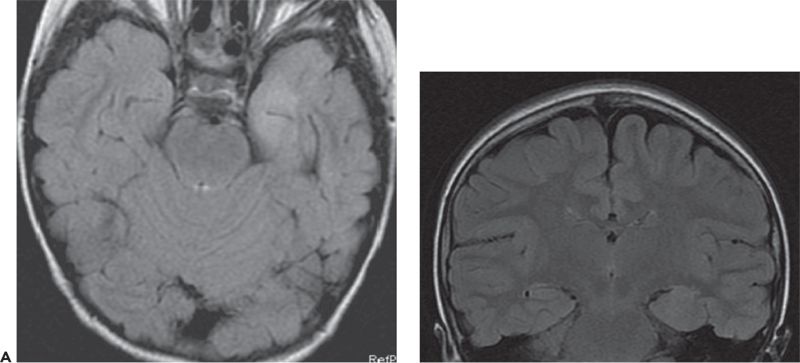
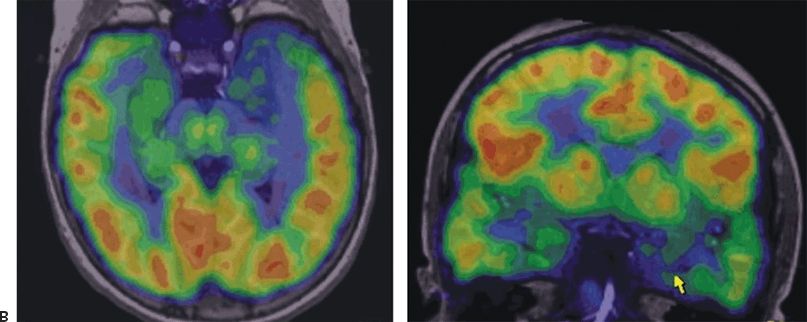
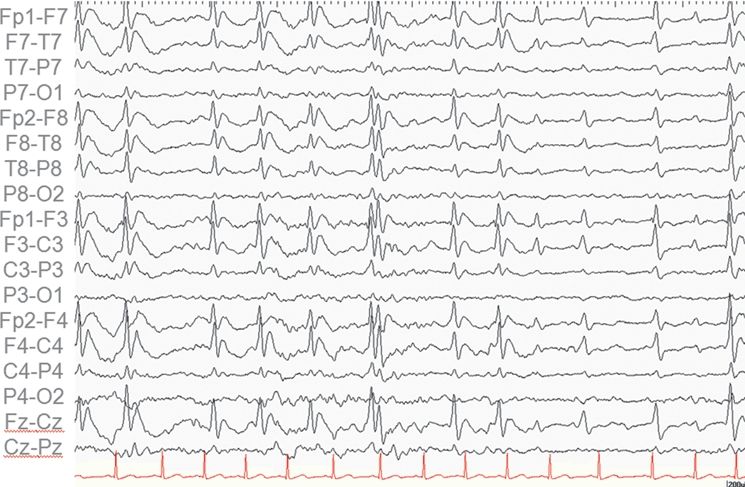
Figure 89.3. Case 3. (All images are of the same patient.) A: MRI showing left anteromedial temporal malformation of cortical development in an 11-year-old girl with history of left temporal lobe seizures from 5 to 7 years of age and then severe epileptic encephalopathy. B: FDG–PET showing severe left anteromedial hypometabolism in the region of the malformation seen in A. C: EEG at 11 years of age showing nonlocalized slow spike-and-wave complexes, nearly continuous during sleep (CSWS pattern). After left anteromedial temporal resection, she was free of seizures with resolution of her epileptic encephalopathy. Postoperative EEG showed no epileptiform discharges while awake or asleep.
A unifying feature in the Cleveland Clinic studies with generalized EEG was the early timing of the occurrence of the lesion seen on MRI, most commonly a malformation of cortical development or encephalomalacia following ischemia, infection, or trauma (41,42). Lesions were congenital or perinatal in 75% of patients and acquired within the first 2 years of life or earlier in 90%. The latest timing of lesion acquisition in the series was at age 5 years. In contrast, the age at evaluation for surgery ranged widely from infancy through young adulthood, with median at 8 years (42). Although mechanisms are unknown, the generalized epileptiform discharges seen later in childhood appear to result from complex early interactions between the epileptogenic lesion and the developing brain (41,42). These studies (41,42) highlight the limitations of scalp video–EEG in children, emphasize the importance of a brain MRI lesion, and show practical difficulties in establishing proof of focal epileptogenicity in some children before surgery. Therefore, in every child, the location of the focal epileptogenic zone must be preoperatively defined, whenever possible, by a convergence of results from clinical examination, video–EEG, anatomic and functional neuroimaging, and other testing, while recognizing that in carefully selected cases with early MRI lesions, generalized EEG patterns may not contraindicate surgery (3,25).
ANATOMIC AND FUNCTIONAL NEUROIMAGING
Neuroimaging is a critical component of surgical strategy at every age. A focal epileptogenic lesion on the MRI seems to indicate a better prognosis for seizure-free outcome. In the Cleveland Clinic pediatric series from 1990 to 1996 (2), 54% of patients were seizure free and 19% had only rare seizures after extratemporal or multilobar resections. In contrast, in the Montreal Neurological Institute pediatric series (43) (excluding tumor cases) during the pre-MRI era between 1940 and 1980, only 27% had few or no seizures after frontal resection. The more favorable results from the Cleveland Clinic may be due to identification of a focal epileptogenic lesion on preoperative MRI in 85% of patients. Almost identical results were reported in an adult series of extratemporal resections performed in Bonn, Germany, from 1987 to 1993, with 54% of patients free of seizures after surgery (44). Seizure-free outcome in that series was significantly more common in lesional than nonlesional cases, with 82% of lesions identified preoperatively by MRI. The absence of MRI localization appears to be an unfavorable prognostic sign, although some patients may have good outcome after EEG-guided cortical resection. The yield of brain MRI, particularly in neocortical frontal and temporal lobe epilepsy, could be enhanced by use of high-resolution imaging with 3-T magnets, specialized protocols with thin sections and surface coil MRI, and experience of the reader (5,45).
PET is also an important neuroimaging tool for pediatric epilepsy surgery. Chugani et al. found that a localized region of hypometabolism may identify focal cortical dysplasia even without abnormal features on MRI (12). This is especially helpful in infants because immature myelination challenges identification of subtle dysgenetic abnormalities of the gray–white junction on routine brain MRI protocols. In a recent study, brain PET was also found to be a useful predictor of seizure outcome after hemispherectomy, with bilateral PET abnormalities being associated with postoperative seizure recurrence (46). PET scans using special tracers have been reported to be useful in some children with tuberous sclerosis (47). Ictal SPECT remains a challenging modality to use in children; however, it has been increasingly used in many centers in selected pediatric cases (32,48,49). Acquisition and interpretation of ictal SPECT in children are complicated as a result of several factors (32). First, interictal SPECT may be difficult to obtain owing to multiple daily seizures in this group of patients. Second, difficulty in promptly recognizing the clinical onset of ictal behavioral changes because of age and coexistent mental retardation may result in a late injection for an ictal SPECT. Third, some extratemporal seizures may be brief and spread rapidly. Fourth, children may require sedation on two occasions to obtain interictal and ictal scans. Newer noninvasive presurgical procedures, such as magnetoencephalography and functional MRI (fMRI), are increasingly being used in children for source localization of interictal spikes (50–53) and mapping of language and motor function (fMRI) using standardized protocols (54,55). However, it remains to be seen if these techniques will independently expand the selection of pediatric surgical candidates, obviate the need for invasive video–EEG recordings, and improve the long-term surgical outcome in children.
ETIOLOGIES AND PATHOLOGIC SUBSTRATES OF EPILEPSY IN PEDIATRIC PATIENTS
Causes of epilepsy differ in children and adults. Figure 89.4 depicts the usual age of onset and common etiologies/pathologic substrates encountered in children with epilepsy. Hippocampal sclerosis, the most common etiologic factor in adult candidates for epilepsy surgery, is uncommon in children. In contrast, in a pediatric epilepsy surgery series from the Cleveland Clinic Foundation (2), hippocampal sclerosis was the cause in only 12% of 62 children (3 months to 12 years of age) and in 15% of 74 adolescents (13 to 20 years of age). Although hippocampal sclerosis may begin in childhood, the typical presentation for surgical evaluation is in early adulthood. When hippocampal sclerosis occurs in pediatric candidates for epilepsy surgery, the clinical and EEG features may be similar to those in adults (56). However, pediatric patients appear to have an especially high incidence of dual pathology with cortical dysplasia in addition to the hippocampal sclerosis (56).
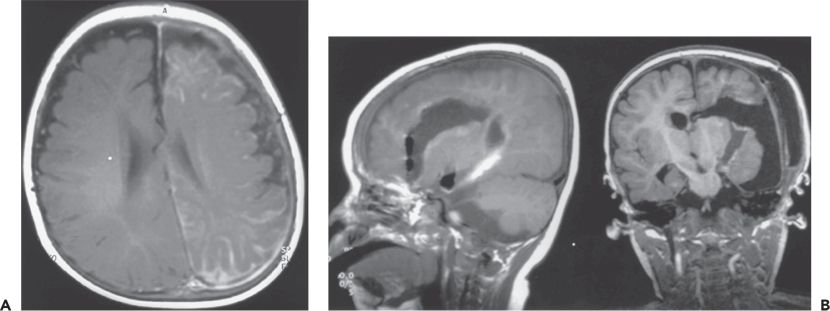
Figure 89.4. Case 4. A:Axial magnetic resonance image at age 12 months, showing Sturge–Weber malformation with left hemispheric atrophy and pial angiomatosis. Starting at age 2 months, seizures occurred once or twice per day characterized by jerking of the right arm or decreased behavioral activity with bilateral eye blinking and lip smacking. Physical examination revealed right hemiparesis, right hemianopia, and developmental delay. Ictal and interictal epileptiform abnormalities were seen in multiple areas of the left hemisphere. B:Sagittal (left) and coronal (right) magnetic resonance images showing the left hemispheric disconnection performed at age 12 months. No seizures occurred during the 8 months since surgery on a reduced dose of antiepileptic medications. Surgery did not worsen neurologic deficits, and the child has progressed developmentally.
In pediatric candidates, the predominant etiologic factors are focal, multilobar, or extensive hemispheric malformation of cortical development (cortical dysplasia) (Figs. 89.1, 89.5, and 89.6) and low-grade tumor (2,3,57). These were the cause of the epilepsy in 57% of adolescents, 70% of children, 90% of infants younger than 3 years in the Cleveland Clinic series (2), and 90% of infants treated surgically in the series of Duchowny et al. (1). Less common causes are vascular malformation, arachnoid cyst, and localized injury due to infarction, trauma, or infection (1,2,57).
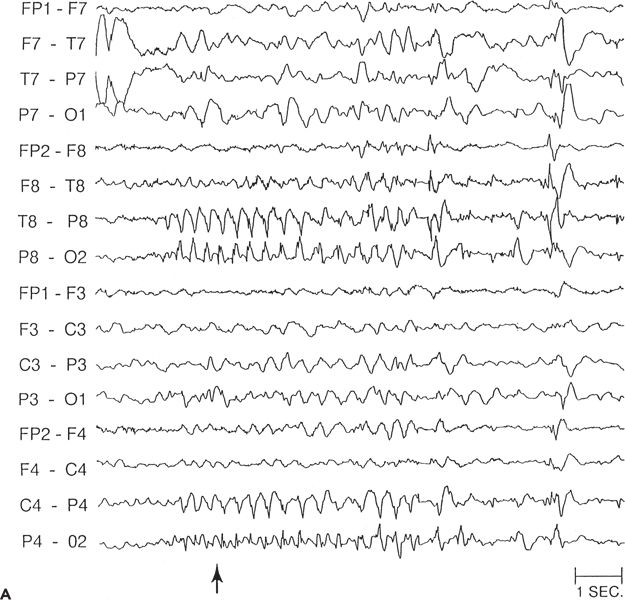
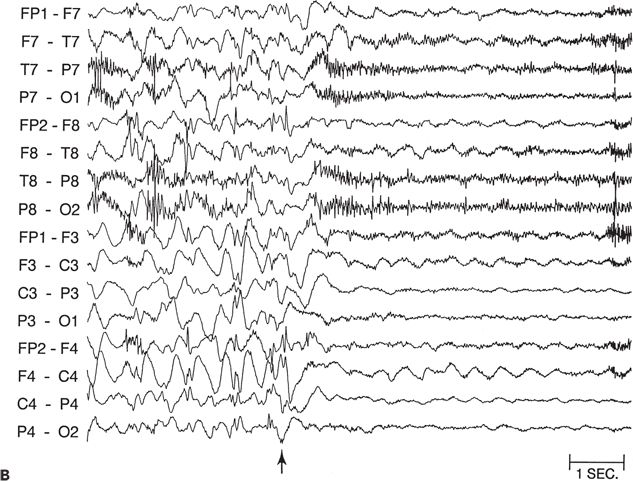
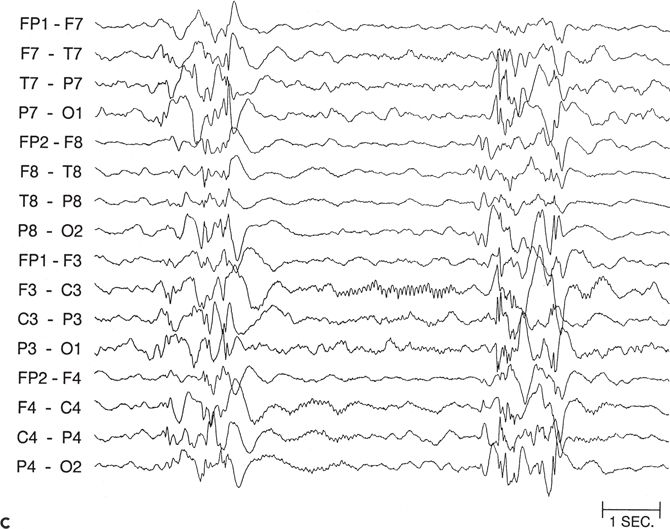
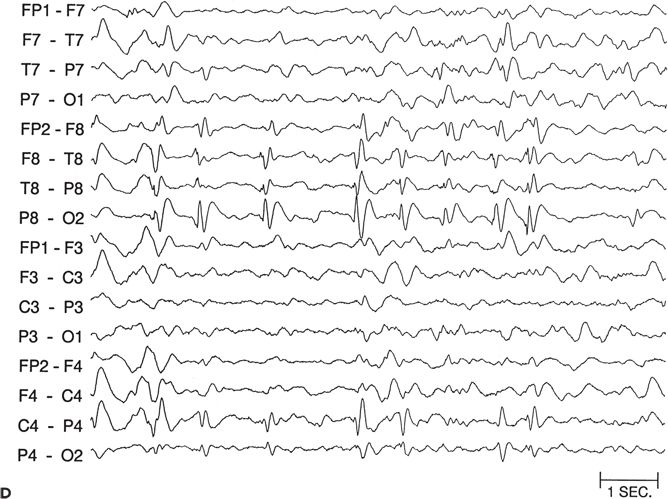
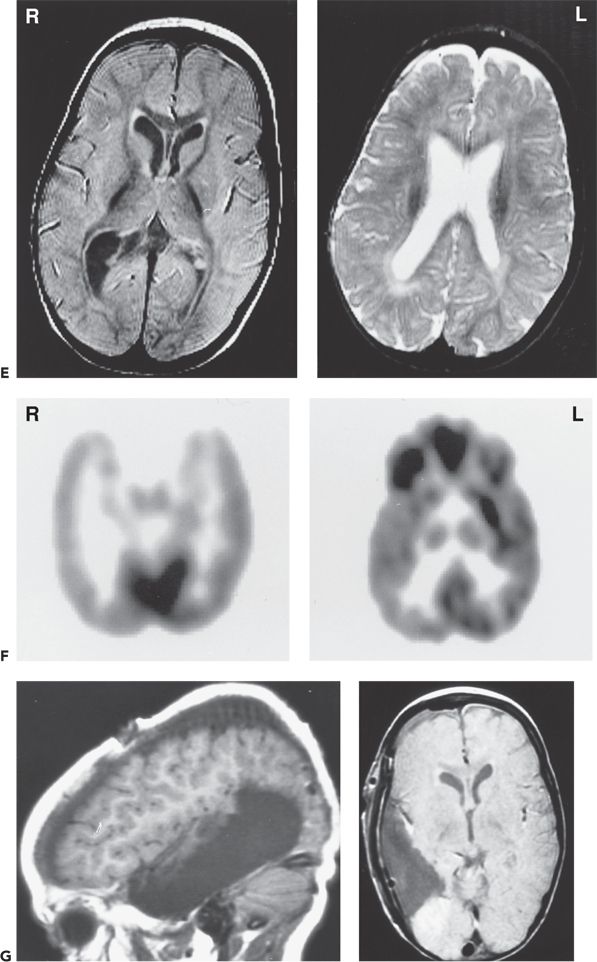
Figure 89.5. Case 5. (All images are of the same patient.) A: Ictal electroencephalogram from a 4.5-month-old infant (patient 2089) showing right parietal onset of a partial seizure (arrow). Seizures began at age 2 months and occurred several times a day. B: Ictal electroencephalogram at age 13 months, showing hypsarrhythmia with diffuse electrodecrement at the onset of an infantile spasm (arrow). Evolution from partial seizures to infantile spasms occurred at age 7 months. The infant had delayed cognitive development and reduced visual attentiveness but no motor deficits. C: Sleep spindles were consistently reduced over the right hemisphere, providing further evidence of right hemisphere dysfunction. D: This carefully selected segment of the interictal electroencephalogram shows that spikes were sometimes predominant over the right parietal region, despite the diffuse hypsarrhythmic pattern during most of the recording. Normal faster frequencies were reduced in that area. E: Magnetic resonance imaging (MRI) at 13 months showed bilateral periventricular leukomalacia, worse in the right parietal region. The findings could have resulted from intrauterine right germinal matrix hemorrhage several weeks before the uneventful term birth. No cortical dysplasia or gyral abnormality was evident on MRI. F: Interictal 2-[18F]fluoro-2-deoxy-d-glucose PET at 13 months showing right parietooccipitotemporal hypometabolism. G: Postoperative MRI showing the right parietooccipitotemporal resection performed at age 15 months. Histopathologic analysis of resected tissue revealed microscopic cortical dysplasia, possibly as a result of disturbance of late neuronal migration at the time of the intrauterine intraventricular hemorrhage. The infant remains free of seizures 17 months after operation and has made “catch-up” developmental progress. (A, B, E, and F are from Wyllie E, Comair Y, Ruggieri P, et al. Epilepsy surgery in the setting of periventricular leukomalacia and focal cortical dysplasia. Neurology. 1996;46:839–841, with permission; A and G are from Wyllie E, Comair YG, Kotagal P, et al. Epilepsy surgery in infants. Epilepsia. 1996;37: 625–637, with permission.)
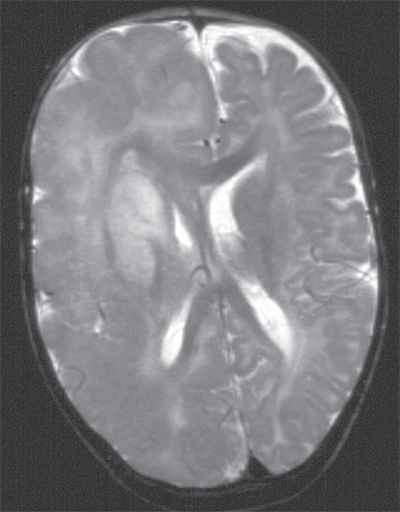
Figure 89.6. T2-weighted sagittal image of “typical” hemimegalencephaly showing diffuse right hemispheric enlargement and dysplasia. Midline shift with bulging of anterior falx to the left and compression of the right lateral ventricle suggests a mass effect as a result of increased volume of the brain parenchyma. Dysplastic changes are diffuse, with thick and disorganized cortex, poor gray–white matter differentiation, and abnormal signal in the white matter. Note that the basal ganglia are also dysplastic with abnormal increased signal.
Hemispheric syndromes are also important etiologies in children undergoing epilepsy surgery in the form of hemispherectomy (46,58). Hemispheric malformations of cortical development like hemimegalencephaly (see Fig. 89.6), Sturge–Weber syndrome, and perinatal unilateral cerebral ischemic insults are the most common etiologic factors in children and adolescents who had hemispheric ablation procedures, with Rasmussen chronic focal encephalitis occurring less frequently (46,58,59).
The age-related differences in etiology result in an age-related spectrum of surgical procedures. Anteromesial temporal resections predominate in adults but not in children. In pediatric series, extratemporal or multilobar resections or hemispherectomies composed 44% of the surgeries in adolescents, 50% in children, and 90% in infants (1,2).
SURGICAL CONSIDERATIONS IN PEDIATRIC PATIENTS
Identification of Candidates: The Timing of Surgery
Critical features of surgical candidacy at any age include intractable epilepsy interfering with quality of life or development, clear identification of a localized epileptogenic zone, and low risk for new postoperative neurologic deficits. However, for each of these factors, age-related issues must be considered in light of results from an extensive presurgical evaluation. The risk of proceeding with surgery must be weighed against the risk of continuing with uncontrolled seizures treated medically. If careful analysis yields a favorable risk–benefit ratio for surgery, then the available data suggest that it is appropriate to proceed regardless of age.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








