Figure 77.1. EEG burst suppression pattern (HF 70 Hz, LF 1 Hz SEN 5 μV/mm) [54].
Burst suppression pattern is used as a clinical tool to avoid over-medicating when the neurologic exam is obliterated by anesthetic medications. This EEG pattern has often been suggested as a goal for therapy in refractory SE, but achieving burst-suppression is neither necessary nor sufficient for the control of SE. Therefore, the goal should be seizure suppression; this is usually maintained for 12 to 24 hours, and then decreased by some arbitrary degree, such as 50%, for several hours while observing for seizure recurrence. Simultaneously, an anticonvulsant or an appropriate combination is added, with the goal of maintaining a seizure free state once barbiturate is cleared. Continuous EEG monitoring is required during this weaning process, as recurrence of seizure activity prompts a return to the previous dose with adjustments in doses or types of the other anticonvulsants [21].
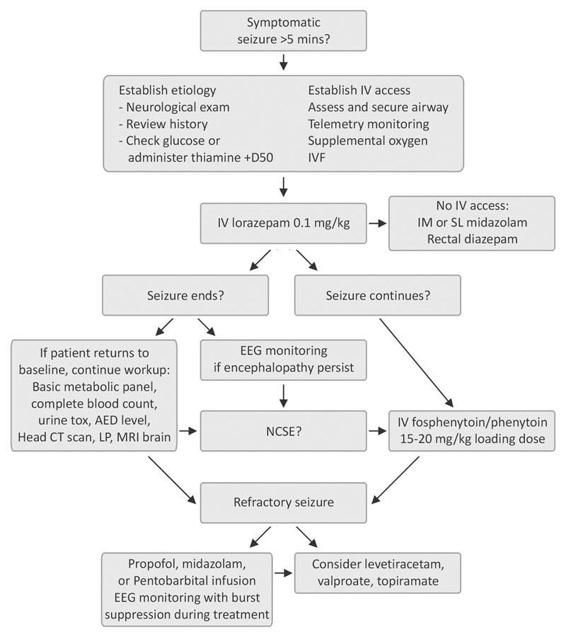
Figure 77.2. Management of status epilepticus.
Midazolam infusion in the setting of refractory SE offers a favorable pharmacokinetic profile [40]. One small study showed rapid control of seizure that had failed multiple medications, including diazepam, lorazepam, phenytoin, with or without phenobarbital, with minimal hypotension [41]. In critically ill patients, midazolam can accumulate, especially as increasingly higher doses of midazolam are usually required to maintain burst suppression. Some studies have shown that there may be lower mortality in a select group of patients treated with a midazolam infusion compared to those treated with a propofol infusion [42].
Propofol has a very short half life in comparison to the alternatives discussed for refractory SE treatment. Additionally it is highly lipid soluble and therefore fast acting in CNS. Several studies and reviews have demonstrated its effectiveness in treating refractory GCSE and NCSE [43-46]. Some recent studies have suggested an increased mortality associated with propofol infusion [41], but others have not supported this trend when compared to pentobarbital or midalozam at anesthetic doses for the treatment of SE [46]. Care must be taken to not discontinue propofol abruptly, as this may be associated with recurrent seizures. Hypotension is more common with propofol than with midazolam. Triglyceride levels must be monitored during prolonged infusion as development of hypertriglyceridemia may prompt discontinuation of propofol infusion. The clinician should observe carefully for development of the the propofol infusion syndrome, which may manifest as either a metabolic acidosis or rhabdomyolysis. If either of these occur, propofol should be discontinued and another agent substituted.
77.7 Conclusions
In the ICU, mortality associated with SE is high, and irreversible brain damage ensues quickly. The detection and diagnosis of SE, along with identification of its etiology, is crucial for clinicians at all stages of this condition, as the goal must be termination of seizure as soon as possible. A systematic yet aggressive approach to the treatment of SE is recommended. The clinician must be prepared to escalate rapidly to more potent anesthetic medications while providing basic life support measures, until either clinical or electrographic end of seizure is apparent.
References
1. DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of SE in Richmond, Virginia. Neurology 1996; 46: 1029-35
2. Bassin S, Smith TL, Bleck T. Clinical review: SE. Crit Care 2002; 6: 137-42
3. Krumholz A, Sung GY, Fisher RS, et al. Complex partial SE accompanied by serious morbidity and mortality. Neurology 1995; 45: 1499-504
4. Walker MC. Treatment of nonconvulsive SE. Int Rev Neurobiol 2007; 81: 287-97
5. Scott RC, Surtees RA, Neville BG. SE: pathophysiology, epidemiology, and outcomes. Arch Dis Child 1998; 79: 73-7
6. Treatment of convulsive SE. Recommendations of the Epilepsy Foundation of America’s Working Group on SE. JAMA 1993; 270: 854-9
7. Nevander G, Ingvar M, Auer R, et al. SE in well-oxygenated rats causes neuronal necrosis. Ann Neurol 1985; 18: 281-90
8. Lowenstein DH, Bleck T, Macdonald RL. It‘s time to revise the definition of SE. Epilepsia 1999; 40: 120-2
9. Drislane FW. Presentation, evaluation, and treatment of nonconvulsive SE. Epilepsy Behav 2000; 1: 301-14
10. Kaplan W. Nonconvulsive SE. Semin Neurol 1996; 16: 33-40
11. Vespa PM, Nuwer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg 1999; 91: 750-60
12. DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive SE after the control of convulsive SE. Epilepsia 1998; 39: 833-40
13. Jordan KG. Continuous EEG and evoked potential monitoring in the neuroscience ICU. J Clin Neurophysiol 1993; 10: 445-75
14. Abend NS, Dlugos DJ. Nonconvulsive SE in a pediatric ICU. Pediatr Neurol 2007; 37: 165-70
15. Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive SE in comatose patients. Neurology 2000; 54: 340-5
16. Fountain NB, Lothman EW. Pathophysiology of SE. J Clin Neurophysiol 1995; 12: 326-42
17. Lothman E. The biochemical basis and pathophysiology of SE. Neurology 1990; 40(5 Suppl 2): 13-23
18. Kaplan W. No, some types of nonconvulsive SE cause little permanent neurologic sequelae (or: “the cure may be worse than the disease”). Neurophysiol Clin 2000; 30: 377-82
19. Kaplan W. Prognosis in nonconvulsive SE. Epileptic Disord 2000; 2: 185-93
20. Abou Khaled KJ, Hirsch LJ. Advances in the management of seizures and SE in critically ill patients. Crit Care Clin 2006; 22: 637-59; abstract viii
21. Mirski MA, Varelas N. Seizures and SE in the critically ill. Crit Care Clin 2008; 24: 115-47
22. Aminoff MJ, Simon R. SE. Causes, clinical features and consequences in 98 patients. Am J Med 1980; 69: 657-66
23. Towne AR. Determinants of mortality in SE. Epilepsia 1994; 35: 27-34
24. DeLorenzo RJ, Towne AR, Pellock JM, et al. SE in children, adults, and the elderly. Epilepsia 1992; 33 (Suppl 4): S15-25
25. Shinnar S, Pellock JM, Berg AT, et al. Short-term outcomes of children with febrile SE. Epilepsia 2001; 42: 47-53
26. Lowenstein DH, Alldredge BK. SE at an urban public hospital in the 1980s. Neurology 1993; 43(3 Pt 1): 483-8
27. Treiman DM. Pharmacokinetics and clinical use of benzodiazepines in the management of SE. Epilepsia 1989; 30(Suppl 2): S4-10
28. Prensky AL, Raff MC, Moore MJ, et al. Intravenous diazepam in the treatment of prolonged seizure activity. N Engl J Med 1967; 276: 779-84
29. Leppik IE, Derivan AT, Homan RW, et al. Double-blind study of lorazepam and diazepam in SE. JAMA 1983; 249: 1452-4
30. Fountain NB, Adams RE. Midazolam treatment of acute and refractory SE. Clin Neuropharmacol 1999; 22: 261-7
31. Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive SE. Veterans Affairs SE Cooperative Study Group. N Engl J Med 1998; 339: 792-8
32. Fakhoury T, Chumley A, Bensalem-Owen M. Effectiveness of diazepam rectal gel in adults with acute repetitive seizures and prolonged seizures: a single-center experience. Epilepsy Behav 2007; 11: 357-60
33. McIntyre J, Robertson S, Norris E, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet 2005; 366: 205-10
34. Knapp LE, Kugler AR. Clinical experience with fosphenytoin in adults: pharmacokinetics, safety, and efficacy. J Child Neurol 1998; 13 (Suppl 1): S15-8; discussion S30-2
35. Browne TR, Szabo GK, McEntegart C, et al. Bioavailability studies of drugs with nonlinear pharmacokinetics: II. Absolute bioavailability of intravenous phenytoin prodrug at therapeutic phenytoin serum concentrations determined by double-stable isotope technique. J Clin Pharmacol 1993; 33: 89-94
36. Twyman RE, Rogers CJ, Macdonald RL. Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann Neurol 1989; 25: 213-20
37. Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med 1999; 341: 485-9
38. Shaner DM, McCurdy SA, Herring MO, et al. Treatment of SE: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology 1988; 38: 202-7
39. Bleck T. Critical care of the patient in SE. In: Wasterlain C (ed.) SE. Cambridge, MA: MIT Press, 2006; pp. 607-13
40. Bleck T. ICU management of patients with SE. Epilepsia 2007; 48(Suppl 8): 59-60
41. Kumar A, Bleck T. Intravenous midazolam for the treatment of refractory SE. Crit Care Med 1992; 20: 483-8
42. Prasad A, Worrall BB, Bertram EH, et al. Propofol and midazolam in the treatment of refractory SE. Epilepsia 2001; 42: 380-6
43. Begemann M, Rowan AJ, Tuhrim S. Treatment of refractory complex-partial SE with propofol: case report. Epilepsia 2000; 41: 105-9
44. Brown LA, Levin GM. Role of propofol in refractory SE. Ann Pharmacother 1998; 32: 1053-9
45. Campostrini R, Bati MB, Giorgi C, et al. Propofol in the treatment of convulsive SE: a report of four cases. Riv Neurol 1991; 61: 176-9
46. Claassen J, Hirsch LJ, Emerson RG, et al. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 2002; 43: 146-53
47. Knake S, Gruener J, Hattemer K, et.al. Intravenous Levetiracetam in the treatment of benzodiazepine refractory status epilepticus. J Neurol Neurosurg Psychiatry 2008; 79: 588-9
48. Möddel G, Bunten S, Dobis C, et al. Intravenous levetiracetam: a new treatment alternative for refractory status epilepticus. J Neurol Neurosurg Psychiatry 2009; 80: 689
49. Eue S, Grumbt M, Müller M, et al. Two years of experience in the treatment of status epilepticus with intravenous levetiracetam. Epilepsy Behav 2009; 15: 467
50. Rüegg Y, Naegelin M, Hardmeier DT, et al. Intravenous levetiracetam: treatment experience with the first 50 critically ill patients. Epilepsy & Behavior 2008; 12: 477-80
51. Aiguabella M, Falip M, Villanueva V, et. al. Efficacy of intravenous levetiracetam as an add-on treatment in status epilepticus: A multicentric observational study. Seizure: European Journal of Epilepsy 2011; 20: 60-4
52. Alvarez V, Januel JM, Burnand B, et. al. Second-line status epilepticus treatment: comparison of phenytoin, valproate, and levetiracetam. Epilepsia 2011; 52: 1292
53. Philippe ER, Andrea P, Soheil Z, et al. Science review: Carnitine in the treatment of valproic acid-induced toxicity – what is the evidence? Crit Care 2005; 9: 431-40
54. Venkataraman V, Wheless JW. Safety of rapid intravenous infusion of valproate loading doses in epilepsy patients. Epilepsy 1999; 35: 147-53
55. Gilad R, Izkovitz N, Dabby R, et al. Treatment of status epilepticus and acute repetitive seizures with i.v. valproic acid vs phenytoin. Acta Neurol Scan 2008; 118: 296-300
56. Towne AR, Garnett LK, Waterhouse EJ, et al. The use of topiramate in refractory status epilepticus. Neurology 2003; 60:333
57. Sinclair DB, Campbell M, Byrne P, et. al. EEG and long-term outcome of term infants with neonatal hypoxic-ischemic encephalopathy. Clin Neurophysiol 1999; 110: 655-9
58. Bensalem MK, Fakhoury TA. Topiramate and status epilepticus: report of three cases. Epilepsy Behav 2003; 4: 757
59. 58. Reuber M, Evans J, Bamford JM. Topiramate in drug-resistant complex partial status epilepticus. Eur J Neurol 2002; 9: 111
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






