CHAPTER 5 Stem Cell Biology in the Central Nervous System
In a series of papers published in the 1960s, Joseph Altman and colleagues reported that certain regions of the rat brain contained dividing cells capable of generating progeny with a neuronal morphology.1 Evidence for cell proliferation in the rat and mouse already existed,2–4 but conventional wisdom at the time was that the adult mammalian brain was completely incapable of regeneration and that neurons were formed only during development. Because technical limitations made verifying the neuronal nature of cells difficult, Altman’s discoveries were met with great skepticism. Decades later, continued research and technical progress have led to unambiguous demonstration of adult neurogenesis. This chapter considers the nature of the neural stem cells (NSCs) responsible for the generation of new neurons and glia in the adult central nervous system (CNS), their role in certain tumors, and the potential cell-based therapies that they represent.
Neurogenesis: Location and Function
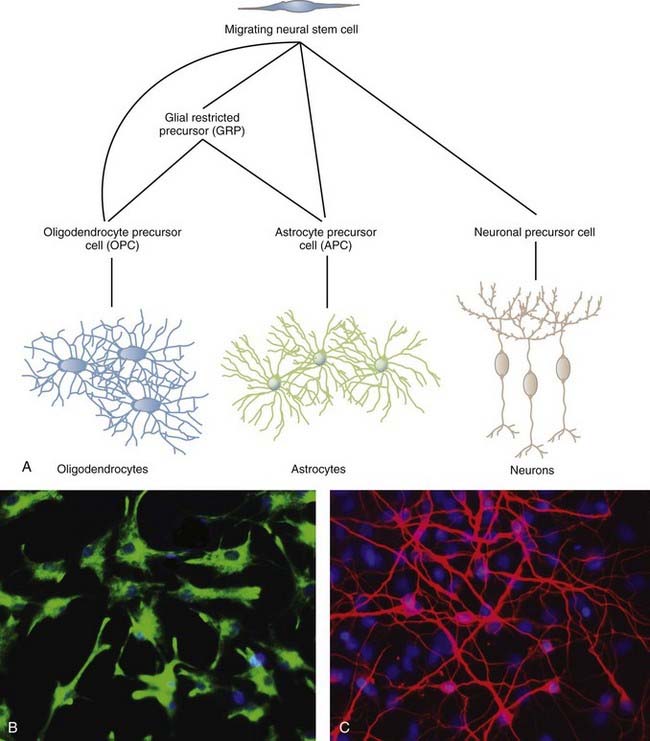
NSCs can give rise to the three major cell types of the CNS: neurons, oligodendrocytes, and astrocytes (Fig. 5-1). The two most studied sites of NSC activity are the subventricular zone (SVZ) and the dentate gyrus of the hippocampus. The bulk of our knowledge of neurogenesis comes from rodent studies, but populations of NSCs have been identified and studied in humans, as discussed later.
The Subventricular Zone
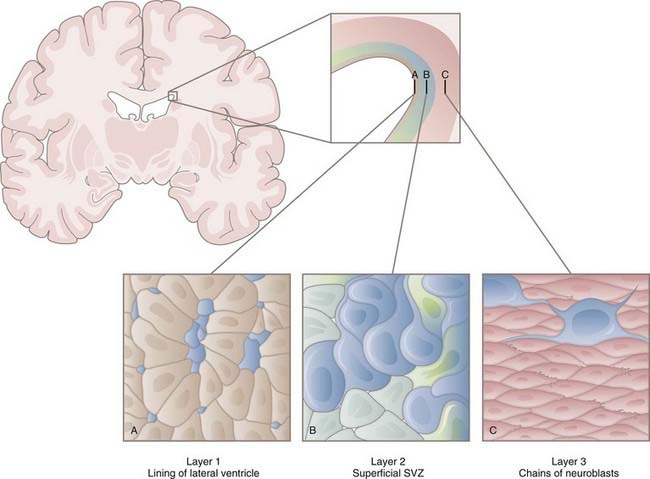
In the early 1990s, Reynolds and Weiss discovered cells in the adult mouse brain that proliferated and differentiated into neurons and astrocytes.5 These cells were also immunopositive for nestin, an intermediate filament of neuroepithelial stem cells prevalent before differentiation. These results led to identification of the SVZ as one of the neurogenic niches present in the adult brain. Within the SVZ is a layer of proliferative cells along the lateral aspect of the lateral ventricle (Fig. 5-2). These cells express glial fibrillary acidic protein (GFAP), a classic marker for astrocytes, and polysialylated neural cell adhesion molecule (PSA-NCAM). Separating the GFAP+/PSA-NCAM+ cells from the ventricular lumen is a layer of ependymal cells, with most of the astrocytes extending minute apical processes that directly contact the ventricle.6 Astrocytes from the SVZ differentiate into immature neurons called neuroblasts that travel via a migratory pathway to the olfactory bulb, where they differentiate into neurons.7–9 This pathway is known as the rostral migratory stream (RMS). The astroglial cells of the SVZ also generate astrocytes, oligodendrocyte precursor cells (OPCs, discussed later), and myelinating oligodendrocytes in response to chemical demyelinating lesions.10–13 This multipotential nature of SVZ astrocytes earns them the classification of NSCs.
Models of the cytoarchitecture of the SVZ have been in a state of flux, but the basic format has been determined. The primary precursors in vivo are the slowly dividing astrocytes mentioned earlier, called type B cells. B cells divide asymmetrically, which means that mitosis results in two different daughter cells. One is an SVZ astrocyte, just like the parent cell. The other is a short-lived transit-amplifying cell called a type C cell. C cells are antigenically distinct from B cells in that C cells express neither GFAP nor PSA-NCAM. After a brief period of increased mitotic activity, C cells ultimately give rise to migrating neuroblasts (type A cells). The neuroblasts travel from the SVZ via the RMS to the olfactory bulb, where they generate two types of inhibitory interneurons: granule cells and periglomerular neurons. C and A cells in the SVZ and subgranular zone (SGZ) can be identified by their expression of the microtubule-associated protein doublecortin14 and can be used as a marker to reflect levels of neurogenesis.15 The transcription factor Tbr2 is also expressed in early postmitotic neurons and their intermediate progenitors.16
In addition to their lineage relationships (i.e., B cells giving rise to C cells giving rise to A cells), all three types are closely associated spatially. As neuroblasts migrate toward the olfactory bulb, they coalesce to form a network of chains moving rostrally.17,18 They do so through cellular tunnels consisting mainly of type B cells with occasional C cells interspersed among them.
Newly born neurons in the adult contend with markedly different circumstances than do those of the embryonic brain. Adult neuroblasts migrate through more intricate terrain, frequently over longer distances. They do so first in a tangential fashion toward the olfactory bulb and then radially away from the RMS.19 To execute this switch, the neuroblasts must detach from the migrating chain, a process regulated by the extracellular matrix protein tenascin-R.20 Tangential and radial migration combined takes place in less than a week, after which the cells must integrate into fully functional circuits. For this reason, it is likely that the differentiation patterns do not simply recapitulate development.21 Soon after migration, the neurons display spontaneous activity and, over the course of the next 5 to 10 days, spiking activity. Overall, it takes weeks for cells to be born in the SVZ, migrate rostrally in the RMS, migrate radially after reaching the olfactory bulb, and differentiate and fully integrate into the existing neuronal circuitry. Even then, a large fraction of the cells (approximately 50%) will not survive without a sufficient level of sensory input.
In early studies of adult neurogenesis, some researchers suggested that the ependymal cells in the periventricular region represent the NSC population.22 Indeed, NSCs have been shown to contain a prominent cilium, although smaller and more specialized than that of the motile cilia characteristic of brain ependyma. However, studies involving antimitotic drugs and viral labeling, among others, strongly suggest that it is in fact the type B cells that represent the NSC population, and this is the generally accepted view.23 Some work suggests that one of the roles of ependymal cells in adult neurogenesis is to produce noggin, an antagonist of bone morphogenetic proteins (BMPs).24 Because BMPs are known to inhibit neurogenesis, as well as induce astrogliogenesis, noggin production by ependymal cells may be a means to maintain the neurogenic niche. Although the presence of the SVZ in humans is clear (reviewed later), existence of the RMS is controversial.25,26
The Subgranular Zone
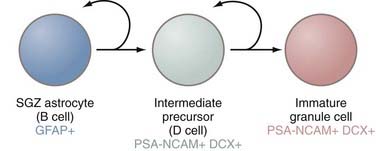
The second principal site of adult neurogenesis is located in the hippocampal formation in a region known as the SGZ (Fig. 5-3). The SGZ lies between the dentate gyrus and the hilum of the hippocampus. In this region of the brain, the primary precursors of new neurons are, as in the SVZ, astrocytes.27 These astrocytes are also referred to as B cells, but much of the rest of the terminology for the SGZ cytoarchitecture differs from that of the SVZ. In the hippocampus, B cells give rise to a set of immature D cells that divide less frequently and are smaller and more differentiated than the C cells of the SVZ. Because D cells are less mitotic than transit-amplifying cells, the increase in production of these intermediates is probably restricted. The D cells then generate the excitatory granule neurons that migrate to the granule cell layer of the hippocampus. These newly formed neurons integrate into a circuitry that is relatively close to their astrocytic forebears, in contrast to the distances that cells born in the SVZ must migrate to reach their ultimate destination in the olfactory bulb. These fully differentiated neurons then extend axonal projections called mossy fibers to hippocampal region CA3, a subfield implicated in learning and memory processes.28,29 The newly formed neurons are integrated into the hippocampal circuitry within a week and form excitatory synaptic connections with both interneurons and CA3 pyramidal cells.30 As in the SVZ, the various cell types can be distinguished on the basis of expression of certain molecular markers. Although SGZ astrocytes express GFAP, D cells and their progeny (i.e., neurons) do not and instead express PSA-NCAM.
The rate at which the hippocampus produces new neurons varies considerably, depending on age, internal factors such as neurotransmitter levels, and external factors such as exercise, stress, and sleep.31 Thousands of new cells may be produced each day, but it is only a very small percentage that ultimately survive, fully differentiate, and integrate into neural networks.
Regulation of Neurogenesis
Several factors have been shown to regulate the birth and integration of new neurons in rodents, including environmental cues, learning-related stimuli, and neuronal activity. For example, postnatal unilateral olfactory deprivation results in a significant reduction in bulb volume, an effect that is reversed with sufficient restoration of stimulation.32 Furthermore, exposure of a mouse to a complex odor environment results in both an increase in incorporation of newborn neurons and enhancement of short-term olfactory memory.33,34 Other physiologic states such as pheromone stimulation and pregnancy also modulate olfactory bulb neurogenesis.35,36 Additional stimuli regulating neurogenesis include exercise, learning, memory, environment, and stress.37 Although most of these stimuli are positive regulators, stress and the associated changes in corticosteroid levels result in a decline in neurogenesis and memory.38 For instance, stress in adult marmoset monkeys results in a decrease in the number of proliferating granule cell precursors in the dentate gyrus.39
Neurogenesis in the hippocampus is also subject to activity-dependent regulation. Because of the diversity of input to and complexity of connections in the dentate gyrus,40,41 this control may be more elaborate in the SGZ than in the SVZ. One of the key functions of the dentate gyrus is the formation of distinct representations of contexts, places, and episodes,42 a role that may render the region sensitive to the environment or cortical activity (or to both). The dentate gyrus, as part of the limbic system, also modulates emotional processes such as stress and depression.43 Consistent with these roles, hippocampal neurogenesis is regulated by the environment, cognitive and emotional processes, and exercise.44–46
On the cellular and molecular levels, γ-aminobutyric acid and glutamate exert numerous effects on neurogenesis, including effects in the realms of proliferation, survival, and integration.47 Additionally, a plethora of growth factors and extracellular matrix components influence the birth and maturation of new neurons in an activity-dependent manner.
Astrocytes as Stem Cells
Current evidence suggests that B cells are derived from radial glial cells, the principal NSC in the embryonic mammalian forebrain.48 Both are NSCs and occupy the same periventricular region of the brain, and both contact the ventricle proper via an apical process. Radial glia also possess a basal process that extends to the pial surface, and some adult SVZ GFAP+ cells also have such a process.
On closer inspection, it turns out that astrocytes are in some ways ideally suited to fulfill the role of primary progenitor.49 Their numerous processes contact many cell types and the basal lamina of blood vessels, and interastrocyte connections exist in the form of gap junctions. These structural features poise astrocytes to integrate signals from a variety of sources to effectively regulate the stem cell niche. Astrocytes are also able to produce signals that drive neurogenesis in vitro.50,51
Parenchymal astrocytes in regions other than the SVZ and SGZ do not appear to be neurogenic in vivo, although astrocytes derived from the cortex, cerebellum, and spinal cord in the first two postnatal weeks can all give rise to neurospheres in vitro.52
Functional Significance of Neurogenesis
A growing body of evidence points to neurogenesis as a critical element in an array of adult brain functions. NCAM-deficient mice show a 40% reduction in bulb size that is restricted to the granule cell layer and results in impaired odor discrimination.53 Antidepressants increase neurogenesis in the hippocampus, but only after chronic, not acute treatment.54 An abundance of evidence suggests that adult neurogenesis is directly involved in learning and memory processes and demonstrates plasticity distinct from that occurring at the level of the synapse.
Gliogenesis
Although much of the focus thus far has been on the generation of new neurons, adult gliogenesis merits attention as well. Cells capable of differentiating into astrocytes, oligodendrocytes, or both are termed glial progenitors and are widespread; they constitute anywhere from 3% to 9% of all cells in the adult CNS.55 Indeed, although only select regions of the adult brain are neurogenic, it appears that much of the brain and spinal cord are gliogenic. The transcription factor Olig2 is an important molecular signature for gliogenesis inasmuch as this marker is found to oppose neurogenesis in the SVZ56 but is an important determinant of glial cell fate.57
Recent data have established that significant myelin replacement occurs and is particularly associated with aging.58,59 Hence, at a minimum, there exist glial progenitor cells that serve a homeostatic function for myelin. However, the designation “glial progenitor” is somewhat broad and includes several cell populations identified by potentiality or expression of surface markers (or both), the most common of which are the chondroitin sulfate proteoglycan NG2, the platelet-derived growth factor receptor PDGFR-α, and the cell surface ganglioside A2B5.55,60 The fact that cells can acquire, lose, and reacquire expression of each marker complicates determining whether cells expressing these individual markers represent identical, partially overlapping, or distinct populations.
Regardless of which marker (NG2, PDGFR-α, or A2B5) is chosen to identify them, OPCs have repeatedly been identified in the adult human CNS.61–64 OPCs are as abundant as other glial cells in both white and gray matter and account for up to 3% of cells in adult human subcortical white matter. In addition, OPCs give rise to multipotent neurospheres and can differentiate into oligodendrocytes in vitro. However, although glial progenitors are multipotent, they appear to lack the self-renewal characteristics of adult NSCs and thus have limited capacity to divide. The true parent or stem cell of the widespread glial progenitor remains to be established and is an important avenue of research with considerable clinical potential.
NG2+ Cells
The proteoglycan NG2 is expressed by a population of cells in the adult CNS that are often referred to as “oligodendrocyte progenitor cells” and sometimes as “polydendrocytes” or “synantocytes.”65 It is likely that NG2 marks multiple cell populations, one with characteristic progenitor qualities and the other a distinct form of differentiated glial cells. For example, cells isolated by NG2 can be induced to form not only oligodendrocytes but also astrocytes and neurons in vivo.66–68 However, when analyzed by electron microscopy, NG2+ cells superficially resemble astrocytes but lack intermediate filaments and possess other cytologic features that distinguish them from astrocytes.69 These cells also lack markers for mature oligodendrocytes, astrocytes, neurons, and microglia,55,60,70–75 which has led some to refer to NG2+ cells as a fourth type of neuroglial cell.69
Aside from their potential role in maintaining myelination during aging, the role of the highly prevalent OPCs in the intact adult CNS is unclear. NG2+ cells, particularly the polydendrocyte morphotype, probably have physiologic roles unrelated to myelination because their processes contact both the nodes of Ranvier76 and blood vessels.77 A subpopulation of NG2+ cells also forms synaptic junctions with multiple types of neurons,78,79 thus representing a putative mechanism for neurons to influence the behavior of progenitors so that they can be adapted to neuronal needs. Finally, OPCs mount an impressive proliferative response, change morphologies, and differentiate into myelinating oligodendrocytes whenever demyelination or inflammation occurs in the CNS.60,80–84
Platelet-Derived Growth Factor Receptor-α
PDGFR-α is also frequently used to identify cells with OPC features.85,86 Mice null for PDGF-A demonstrate the importance of this polypeptide in that knockout results in a reduction or even elimination (depending on the region) of PDGFR-α+ progenitors and oligodendrocytes.87 Depletion of these cells also results in severe hypomyelination and a tremor phenotype. Conversely, overexpression of PDGF results in excessive and ectopic oligodendrocyte production, although these redundant oligodendrocytes are eliminated via apoptosis.88 Furthermore, adult OPCs generally divide slowly in culture unless given PDGF, levels of which are upregulated after injury.89 Thus, OPCs may be in a relatively quiescent state until triggered by signals generated by injury, inflammation, or aging.
Because PDGFR-α and NG2 are used to mark the same cell (i.e., the OPC), one might well wonder whether the two are functionally related in any way. In fact, NG2 is required for effective signal transduction of PDGF at the level of the receptor.90,91 The two molecules are highly colocalized in certain populations, are upregulated in response to treatment with basic fibroblast growth factor (bFGF), and can form a molecular complex.90 PDGFR-α+/NG2+ cells can also generate progenitors expressing a third OPC marker, A2B5.
A2B5
A2B5 is a cell surface ganglioside that is also sometimes used to identify glial progenitors. Like the markers already discussed, A2B5 is expressed by oligodendrocytes early in their differentiation and is then downregulated as the cell matures. Recent work suggests that A2B5+ cells arise directly from NG2+ cells.75 A2B5 expression in and of itself is not sufficient for the identification of oligodendrocyte-restricted precursors because this marker is also found on neuronal progenitors and certain types of astrocytes.65 In fact, A2B5 is frequently used to identify a bipotential cell population called O2-A cells, so named for their capability of forming both oligodendrocytes and type 2 astrocytes (although the latter has been demonstrated only in vitro).75,90,92–94
Astrocyte Precursor Cells
In general, astrocyte precursor cells (APCs) are less well characterized than OPCs. This is probably attributable to the fact that until recently, most scientists thought that astrocyte differentiation occurred “by default.”95,96 Studies demonstrating enhanced formation of astrocytes via inhibition of neurogenesis97 or simply absence of proneural transcription factors98 implied that unless a stem cell receives signals otherwise, its fate is astrocytic. This suggests a uniform differentiation pathway. However, given the wide range of functions attributed to astrocytes and the heterogeneity with which they perform these tasks, it now seems simplistic to think that all astrocytes are created equal.
A number of studies have reported astrocyte-restricted precursors, all of which are negative for GFAP expression and are A2B5+ (or derived from A2B5+ cells).99–103 Another common feature of these cells is that nearly all of them require FGF for survival. One putative APC marker, CD44, has been detected in human fetal tissue100 and in certain gliomas (see later). Although early oligodendrocytes and radial glial cells are CD44 negative,102,104 the specificity of this marker for APCs is not quite definite.105
Neural Stem Cells in the Spinal Cord
Self-renewing and multipotent NSCs also exist in the adult spinal cord.22,73,106,107 There is evidence supporting their residence near the central canal,22,108,109 as well as in the white matter parenchyma.73,110,111 Interestingly, NSCs in the spinal cord behave differently from those in the SVZ or SGZ in that they are glial restricted (i.e., they do not give rise to neurons). However, NSCs isolated from the spinal cord are able to generate neurons in vitro111 and when transplanted into the neurogenic dentate gyrus,106 thus indicating that in addition to intrinsic factors, the niche in which NSCs are located is a critical determinant of cell fate. The essential elements of the neurogenic microenvironment are both molecular (cytokines, growth factors, other)112 and cellular, including endothelial cells113,114 and astrocytes.51,115,116
Stem Cell and Progenitor Response to Injury
After brain injury (be it traumatic, ischemic, or chemical), endogenous repair occurs, albeit in a limited fashion.117–119 The neurogenic regions of the brain (i.e., the SVZ and hippocampus) respond to ischemic and kainite insults by increasing proliferation and the number of new neurons that do survive and integrate.120–124 After an ischemic lesion in the striatum, these neurons acquire the phenotype of striatal neurons and their production continues for months after the initial injury,125,126 thus demonstrating the potential for a sustained and specific response.
However, the proliferative response to CNS injury occurs predominantly in glial precursors in the parenchyma.119,127–131 OPCs proliferate, migrate to the site of the lesion, and myelinate in response to a wide range of pathologic states, including trauma,132–136 ischemia,137–140 and demyelination.80,81,141–144 A number of growth factors are upregulated after injury, some of which (e.g., PDGF, FGF) are mitogenic for OPCs. Interestingly, because these factors seem to inhibit terminal differentiation and myelin production,88,145–149 their effects must be counteracted for myelination to occur. Neuronal differentiation in regions other than the SVZ and SGZ is rare and, when it does occur, abortive.150,151 Endogenous NSCs alone and unmodulated are therefore incapable of complete repair, in part because of the microenvironment, as discussed previously. These limitations must be considered when developing therapeutic interventions for CNS injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree