Chapter 176 Stereotactic Radiosurgery for the Treatment of Spinal Metastases
Spinal metastases are estimated to be 20 times more common than primary spine tumors affecting the spine.1 They are reported in as many as 50% of cancer patients and can result in devastating sequelae in 5% to 14%.2–4 Patients with spinal metastases often present with disabling pain, as well as neurologic deficits, as a result of epidural spinal cord compression. Whereas the surgical goal for primary spine tumors such as chordoma and chondrosarcoma is en bloc resection for potential cure, the role of surgery in spinal metastases is generally palliative.3,5 With this in mind, treatment decisions for spinal metastases are made with the intent of resuming systemic therapy as soon as possible for overall disease control or improving quality of life in the final stages of disease.
In North America, over 200,000 new cases of spinal metastases are diagnosed each year, with 20,000 clinical cases of spinal cord compression.6–8 This number is expected to rise as patients live longer with improved response to systemic therapy. These patients have a median overall survival of 7 months, with a range of 3 to 16 months.9–11 Both early detection and appropriate intervention are essential to minimize the sequelae of spinal metastases and maximize patient function and quality of life.12
The principle treatment modalities for solid tumor spinal metastases are surgery and radiation.3,4,13–17 These treatments are generally considered palliative in nature. Typically, most patients have concurrent systemic visceral and/or bone disease at presentation with spinal metastases. Even in the presence of solitary spinal lesions, it is unclear when systemic metastases will develop, providing reason for caution when considering “curative resections” for solitary spinal metastasis.18–20 In terms of other therapeutic options, with few exceptions (e.g., multiple myeloma, lymphoma, breast, and prostate carcinoma), chemotherapy, hormonal therapy, and immunotherapy play a limited role in the treatment of metastatic spine tumors.
Characterization of Pain
Metastatic involvement of the spine can occur anywhere along its axis. These tumors, however, most frequently involve the thoracic spine (70%), followed by the lumbar spine (20%) and cervical spine (10%).5,21 Pain is the most common symptom and can be characterized as either oncologic or mechanical, or a combination of both.19 It is essential to determine which type of pain patients are experiencing as this plays a major role in determining the most effective treatment. Pain that is oncologic or biologic in nature is described as dull, constant, and responsive to steroids. It is often worse at night and in the morning but typically does not worsen during the day with activity. As such, patients often complain of pain during sleep. The diurnal variation is thought to be related to the variable secretion of endogenous steroids, which is why this type of pain is responsive to oral steroid administration in the early stages.19 Though the pathophysiology is uncertain, it is thought to be related to inflammation of tumor within the vertebral body and stretching of the periosteum with tumor growth.
Mechanical pain is associated with activity and worsens with movement.22 In the cervical spine, neck pain that worsens with flexion, extension, and rotation is common and is related to progressive bony destruction. Pain of this type is often suggestive of underlying instability, deformity, or fracture. Rotational pain is suggestive of atlantoaxial instability, whereas pain with flexion and extension is indicative of subaxial pathology. In the thoracic spine, pain is often worse with extension as patients attempt to lie on their back during sleep. Mechanical pain in the lumbar spine is also associated with flexion, extension, and axial loading. Painful radiculopathies may occur as a result of loss of vertebral height and neuroforaminal compression of exiting nerve roots due to either tumor or loss of height with axial loading.
Pain, whether oncologic or mechanical, may be severe enough to make ambulation impossible. Though these patients may have preserved strength in their legs and appear comfortable while supine or on their side, pain may make any movement unbearable. Though a full discussion of the numerous treatment options available for metastatic disease is beyond the scope of this chapter, a number of treatments, including vertebral cement augmentation, surgery, radiation therapy, or SRS, or a combination of these, may play a role in the multimodality treatment of these patients.12 Each patient requires a specifically tailored treatment plan based on clinical and radiographic findings.
Role of Surgery
To fully appreciate the role of SRS in the management of metastatic spine tumors, an understanding of the current role of surgery for metastatic spine disease is required. In 2005, Patchell et al.4 published a prospective randomized trial comparing surgery and conventional external-beam radiation therapy to radiation therapy alone for high-grade spinal cord compression. This study showed that surgery followed by conventional radiation resulted in significant advantages in terms of overall maintenance and recovery of ambulation, continence, narcotic requirements, and survival.
Patients who did not undergo surgery but had radiation upfront had worse outcomes overall than did the surgical group. Fifty-seven percent of ambulatory patients in the radiation arm maintained ambulation for only 13 days compared with 122 days in the surgical arm.4 Nonambulatory patients in the radiation group recovered ambulation in 19% (3/16); however, these patients all recovered ambulation only after crossing over to the surgical arm.4 Essentially no patient recovered ambulation without surgery due to the radiation insensitivity of solid tumor malignancies. Although the study demonstrated the superiority of surgery and conventional radiation therapy to radiation alone in terms of neurologic function, it did not address the durability of tumor control given the relatively short survival of the patients studied.
Despite the study’s limitations, the current recommendation for optimal functional outcomes in patients with high-grade spinal cord compression resulting from solid tumor malignancies or in situations where gross spinal instability exists, is that surgery should be the first-line treatment when possible.4,18
Limitations of Conventional External-Beam Radiation Therapy
Many patients with spinal metastases may have significant medical comorbidities that preclude aggressive surgical treatment when otherwise indicated. For this reason, radiation continues to be the mainstay of treatment for many of these patients. The historical benefits of conventional radiation therapy for treating spinal metastases have been demonstrated in numerous retrospective studies showing improvement or maintenance of neurologic function.11,13–16 Although the Patchell study demonstrated the superiority of surgery, radiation is essential for achieving postoperative local tumor control.23 In a recent review of the literature, Gerszten et al.23 found that the ambulatory rate after conventional radiation was 60% to 80%, pain control was achieved in 50% to 70%, and local tumor control was achieved in 20% to 89% of patients. However, though conventional radiation (usually 30 Gy, in 3 Gy/fraction) provides durable control for the hematologic malignancies and breast carcinoma, the majority of solid tumors demonstrate radioresistance or nondurable long-term tumor control when it is used as either a primary or a postoperative treatment.24 In addition, because treatment plans with conventional radiation often include a margin of one healthy vertebral body above and below the area of concern, this results in a large volume of normal tissue being irradiated.8,25
The variable responsiveness of spinal metastases to radiation was originally reported in a series by Greenberg et al.,26 comparing outcomes of surgery and radiation to radiation alone. Though the surgical approach in this series was mainly laminectomy, thus rendering the surgical results less applicable today with the facile use of advanced decompression and instrumentation techniques, the authors demonstrated marked differences in the response to radiation based on tumor histology. Patients with breast carcinoma and hematologic malignancies showed improved outcomes compared with those with radioresistant tumors such as renal and lung carcinomas.
Maranzano and Latini16 also demonstrated significant differences in the sensitivity of various tumor types to conventional radiation when used as initial treatment. In a study of 275 patients, those with radioresistant tumors such as non–small-cell lung, bladder, and renal cell carcinoma demonstrated significantly less recovery than those with typically radiosensitive tumors, such as breast and hematologic malignancies. Patients with gross instability were excluded from the study. Overall, 98% of patients maintained ambulation but only 60% recovered. Of those who regained ambulation, 70% had radiosensitive tumors. For example, breast carcinoma demonstrated an 80% response rate compared with hepatocellular carcinoma with a 20% response rate. Furthermore, the durability of the response was 10 to 16 months for radiosensitive tumors compared with 1 to 3 months in radioresistant tumors. A number of other studies have also demonstrated this variable response based on tumor histology.27–29
Limited surgical studies have evaluated the utility of conventional radiation as a postoperative adjuvant. Klekamp and Samii30 reported on 101 surgeries, of which 91% were aggressive subtotal or complete resections. All patients underwent postoperative radiation therapy. Local recurrences were 57.9% at 6 months, 69.3% at 1 year, and 96% at 4 years. The primary factor predictive of recurrence was tumor histology.
Although surgery and conventional radiation therapy are the current mainstays of treatment for spinal metastases, the major drawback to this approach is the relatively low radiation tolerance of the spinal cord.31–33 This is particularly relevant in cases of progression or recurrence of metastatic disease following standard radiation therapy. Further conventional radiation at recurrence is typically not an option, and patients who undergo surgery after having previously had radiation are known to do poorly and have increased risk of wound complications and worse functional outcomes.23,33–35
Issues related to tumor radiosensitivity and the need for higher dosing to achieve effective and durable tumor control as well as limited spinal cord radiation tolerance has fueled the development of advanced radiation delivery systems such as SRS to achieve conformal high-dose radiation delivery and durable tumor control.8,23,25,36,37
Stereotactic Radiosurgery
The failure of conventional radiation to achieve long-standing tumor control for radioresistant tumors has limited its modern day application. Though earlier studies have demonstrated success with pain reduction, local disease control, and neurologic improvement in select tumor types, these results do not apply across all histologies.2,23,27,38 As mentioned earlier, one of the main limitations to achieving local tumor control with conventional radiation therapy is the high dose required. However, the ability to deliver this dose is limited due to the adjacent spinal cord, where standard radiation tolerance is considered to be 45 to 50 Gy.31 Though clinical studies continue to improve our understanding of spinal cord tolerance to radiation, the therapeutic index of radiotherapy limits the radiation dose near the spinal cord to such an extent that tumor control is compromised.33,34
SRS has emerged as an advanced image-guided technology, allowing for more targeted and higher radiation dosing to tumors adjacent to organs at risk (OAR) such as the spinal cord. With technological advances in image guidance and radiation delivery platforms, it is now possible to deliver SRS in a highly conformal manner with a steep dose fall-off gradient either as a single fraction or as a hypofractionated regimen (two to five fractions).8,39–44 Such a gradient allows for delivering high doses of radiation within millimeters of vital OAR such as the spinal cord, kidney, and esophagus. This enables the very focused delivery of a potentially cytotoxic tumoral dose that can spare nearby normal tissue.
SRS is increasingly being applied to primary malignant and benign spine tumors; however, its application to spinal metastases represents the largest experience to date.36,42,45,46 The increased application of SRS is further redefining the term radioresistant as tumors traditionally regarded as radioresistant, such as renal cell carcinoma and melanoma, have demonstrated marked responses with durable tumor control following SRS.37 These improved tumor control rates are seen whether used as stand-alone therapy or as a postoperative adjuvant.37,47,48
The emergence and effectiveness of SRS as an instrument to achieve durable local control has also fueled the debate regarding the optimal management strategy for solitary metastatic lesions to the spine, which by some investigators have been considered ideal candidates for en bloc resection for potential cure.17,49,50
Indications and Treatment Planning
The indications for treatment with SRS are not rigidly defined in the literature and are currently evolving based on the experience of several high-volume centers.25,37,43 The most common indication for spine radiosurgery is pain, with 70% to 90% of all patients presenting with severe oncologic pain referable to a corresponding lesion involving one to three levels on imaging. Other indications include upfront treatment for radioresistant histologies, treatment after surgery for residual tumor, impending spinal cord compression, and local disease progression either during observation or after other treatment modalities such as surgery, radiation, and chemotherapy have failed.
The prescribed dose normally takes into account the point maximum dose and volume of spinal cord being irradiated as well as previous radiation exposure to normal tissue. However, there are currently no guidelines for optimal dose and dose constraints. Common dose regimens range from 14 to 24 Gy in a single fraction, or hypofractionated regimens such as 5 Gy in six fractions or 9 Gy in three fractions.25,36,38,40,44,51
Spine Radiosurgery as Monotherapy
The utilization of SRS as monotherapy has several advantages over conventional radiation therapy. With the conformal nature of dose delivery with SRS, vertebral levels adjacent to the treated target are spared from ionizing radiation. This limits the deleterious effect of radiation on whatever viable bone marrow remains. This is particularly important for patients whose hematologic cell counts often decrease as a result of systemic chemotherapy. SRS also allows patients to resume systemic treatments quickly as radiation is usually given in one fraction versus multiple fractions for conventional radiation therapy.12
In one of the largest series to date, Gerszten et al.37 reported on 500 tumors of various histologies treated with high-dose single-fraction radiation at a median dose of 20 Gy (range 12.5–25 Gy) throughout the spine. Pain and radiographic tumor control were achieved in 86% and 90% of cases, respectively. Radiographic tumor control differed based on primary pathology, with breast and lung carcinomas showing 100% radiographic tumor control, compared with renal cell tumors (87%) and melanoma (75%).
SRS allows for the delivery of tumoricidal radiation doses to tumors historically radioresistant to conventional radiation. This response appears to be histology independent. For instance, renal cell carcinoma has traditionally been considered resistant to conventional radiation therapy. Gerszten et al.46 reported 60 cases of renal cell carcinoma treated with single-fraction SRS. The majority (48/60) had progressed despite previous conventional radiation. Treatment doses ranged from 14 to 21 Gy with a mean maximum tumoral dose of 20 Gy, and the median follow-up was 37 months. Pain improved in 34/38 (89%) of patients who presented with oncologic pain, and tumor control was achieved in 7/8 patients who presented with tumor progression. Only 6/60 patients (10%) required surgery for progressive neurologic symptoms after SRS. Despite the high number of reirradiated patients undergoing single-fraction SRS for salvage, no radiation myelopathy or other toxicity was seen in the follow-up period.
Yamada et al.43 reported 101 cases treated with single-fraction SRS predominantly to radioresistant histologies, with the exception of six breast cancer patients. No patient had prior radiation or surgery to the treated area. The treatment paradigm was a dose escalation of 18 to 24 Gy, with the maximum dose to the spinal cord set to 14 Gy. At a median follow-up of 16 months, the overall radiographic control rates were 90%. Seven failures occurred at a median time of 9 months. A statistically significant dose response was demonstrated at 24 Gy compared with 18 Gy. Yamada reanalyzed the data in 248 patients receiving single-fraction radiation, and this dose response difference was maintained at 5-year follow-up. Toxicity was limited to grade 1 and 2 esophageal and skin cancers. No patient experienced myelopathy or functional radiculopathy.
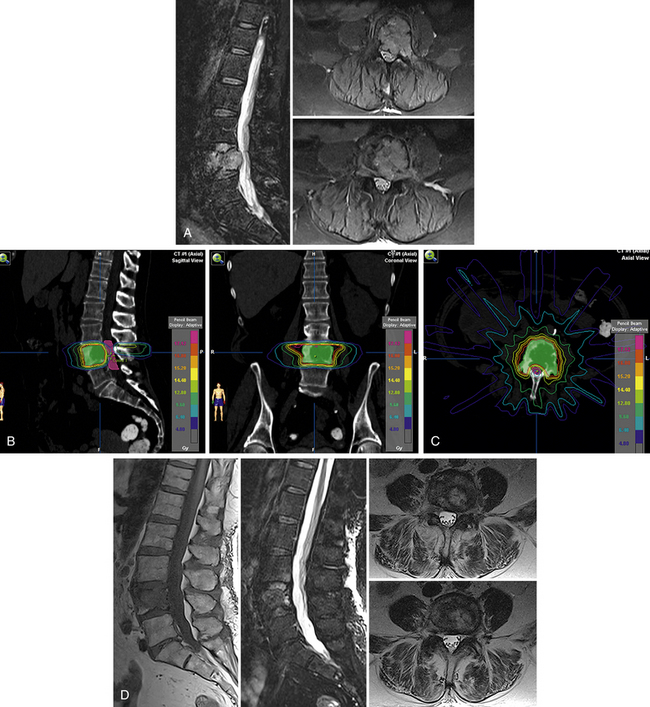
A representative case of a patient with radioresistant histology (renal cell carcinoma) from our own center is illustrated in Figure 176-1. As illustrated in this case, excellent pain control and control of tumor growth by SRS can ensure acceptable quality of life and functional performance in patients with metastases and would avoid the unnecessary morbidity and risk associated with extensive spinal surgery. SRS also allows patients to return home the same day and avoid lengthy hospitalizations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree