Figure 81.1. Intraoperative aspect of SDG/depth electrode implantation in a patient with extratemporal epilepsy and nonlesional MRI. The picture shows a large right-sided temporofrontal craniotomy with placement of SDGs at the dorsolateral convexity of the temporal area. Depth electrodes were implanted in the mesial structures of the temporal lobe (amygdala and hippocampus) and frontal opercular areas.
PRINCIPLES AND INDICATIONS OF SUBDURAL ELECTRODE PLACEMENT
Extraoperative mapping with the subdural method (which includes SDGs and strips) has the main advantage of allowing optimal coverage of the subdural space adjacent cortex with adequate and continuous superficial functional mapping capabilities (13,25–27). A major strength of subdural electrodes consists in the comprehensive anatomical coverage of cortical surfaces, therefore allowing accurate anatomical–electrical and functional mapping of the areas of coverage. Limitations of SDG electrodes include the inadequate/partial/incomplete intrasulcal, deep brain, and interhemispheric coverage and the relative difficulty in multilobar, three-dimensional, and large functional network sampling (28). These characteristics are highlighted in the published results on the successes and failures following SDG implantation (29). The best resective surgical outcomes following SDG implantation are achieved in patients with clear cortical lesions (in particular tumors) and those patients in whom the SDG implantations were placed with the main purpose of functional mapping (12,29). On the other hand, the worst outcomes were seen in patients with no clear lesions on the MRI, nonspecific histopathology, and those who underwent either a sublobar resection or multilobar resections.
These characteristics suggest that the best candidates for extraoperative invasive evaluation with SDG are those patients with clear cortical surface lesion(s) (excluding the interhemispheric, cingulate gyrus, deep sulcal, and mesial frontal/temporal regions), specifically patients undergoing invasive evaluation for electrofunctional/eloquent cortex mapping in the setting of a superficial cortical lesion. Intraoperative ECoG, as compared to chronically implanted SDGs, is a limited option because it usually only provides information restricted to interictal activity. When used in patients under general anesthesia, anesthetic agents may influence EEG activity by altering the thresholds of afterdischarges and motor responses creating a misleading EEG picture (19). Additionally, intraoperative functional mapping often requires a cooperative patient that can tolerate being awake during surgery under local anesthesia. This is particularly difficult in the pediatric population.
LIMITATIONS AND COMPLICATIONS OF SUBDURAL GRID IMPLANTATIONS
The main limitations of SDG include surgical morbidity risks and the spatial/cortical sampling limitations as detailed above. SDG electrodes are foreign bodies that are surgically inserted in the cranial vault, and risks of the procedure include wound infection, flap osteomyelitis, acute meningitis, cerebral edema, and hemorrhage (30–32). Concerns about increased intracranial pressure may reduce the maximal number of electrodes that can be inserted and therefore may lead to incomplete or limited spatial coverage. Other limitations may include the technical challenges that are associated with SDG implantations following a first resective surgery failure (so called “re-do” surgeries). Technical challenges mainly occur due to cortical adhesions in this setting. As stated above, one of the main weaknesses of the subdural methodology is the inability to record from deep cortical areas, as in the depth of sulci, interhemispheric regions (in particular the cingulate gyrus), mesial temporal or frontal structures, and insula/opercular regions (28). Depth electrodes implanted using a non- or semistereotactic technique can partially compensate for the rather incomplete deep/mesial structure coverage with SDG, but as these electrodes are not fully stereotactically implanted, their placement may not be very accurate (29).
THE STEREOELECTROENCEPHALOGRAPHY METHOD
The SEEG method was developed in France by Jean Talairach and Jean Bancaud during the 1950s and has been mostly used in France and Italy as the method of choice for invasive mapping in refractory focal epilepsy (17,33,34). The principle of SEEG is based on AEC correlations with the main aim to conceptualize the 3-dimensional spatiotemporal organization of the epileptic discharge within the brain based mainly on seizure semiology. The implantation strategy is individualized, with electrode placement based on a preimplantation hypothesis that takes into consideration the primary organization of the epileptiform activity and the hypothetical functional epileptic network that may be involved in the propagation of seizures. For these reasons, the preimplantation AEC hypothesis is the most important element in the process of planning for SEEG electrodes placement. If the preimplantation hypothesis is incorrect, the placement of the depth electrodes will be inadequate and the interpretation of the SEEG recordings misleading. The most important characteristic of SEEG methodology is that it enables precise recordings from deep cortical and subcortical structures, multiple noncontiguous lobes, as well as bilateral explorations while avoiding the need for large craniotomies (21,28,35–38).
The SEEG technique was originally described as a multiphase and complex method, using the Talairach stereotactic frame and the double grid system in association with teleangiography (6,39). Despite its long reported successful record, with almost 60 years of clinical use, the technical complexity regarding the placement of SEEG depth electrodes may have contributed to its limited use in centers outside Europe. Taking advantage of new imaging and computational innovations, more modern and less cumbersome methods of stereotactic implantation of depth electrodes can be applied on a routine basis.
PRINCIPLES AND TECHNIQUE OF SEEG IMPLANTATION
The development of an SEEG implantation plan requires the clear formulation of a specific anatomoelectrofunctional hypothesis to be tested. This hypothesis is typically generated during the patient management conference based on the results of various noninvasive tests. At Cleveland Clinic, a final tailored implantation strategy is generated during a separate presurgical implantation meeting. Depth electrodes sample the anatomic lesion (if identified), the more likely structure(s) of ictal onset, the clinically active regions, and the possible electrical pathway(s) of seizure-onset propagation (functional networks). The EZ may correspond to the first clinical sign or may reflect a spread area from a “clinically silent” ictal onset zone within a functional network. For these reasons, a three-dimensional “conceptualization” of the network nodes upstream and downstream from the hypothesized clinical onset region is an essential component of the presurgical implantation strategy. The desired targets are reached using commercially available depth electrodes in various lengths and variable number of contacts, depending on the specific brain region to be explored. The electrodes are implanted using conventional stereotactic technique through 2.5-mm diameter drill holes. Depth electrodes are inserted using orthogonal or oblique orientation, allowing intracranial recording from lateral, intermediate, or deep cortical and subcortical structures in a three-dimensional arrangement, thus accounting for the dynamic, multidirectional spatiotemporal organization of the epileptic pathways.
As part of our routine practice, the patient is admitted to the hospital on the day of surgery. The day before surgery, a stereo contrasted volumetric T1 sequence MRI is performed. Images are then transferred to our stereotactic neuronavigation software (iPlan Cranial 2.6, Brainlab AG, Feldkirchen, Germany) where trajectories are calculated the following day. The day of surgery, while the patient is under general anesthesia, the Leksell stereotactic frame (Elekta, Stockholm, Sweden) is applied using standard technique. Once the patient is attached to the angiography table with the frame, a stereo DynaCT and a three-dimensional digital subtracted angiogram are performed. The preoperative MR images, the stereo DynaCT, and angiographic images are then digitally processed using a dedicated fusion software (syngo XWP, Siemens Healthcare, Forchheim, Germany). These fused images are used during the implantation procedure to confirm the accuracy of the final position of each electrode and to insure the absence of vascular structures along the electrode pathway, which may not be noted on MRI with contrast. Following the planning phase using the stereotactic software, trajectories’ coordinates are recorded and transmitted to the operating room. Trajectories are in general planned in orthogonal orientation in relation to the skull’s sagittal plane in order to facilitate implantation and later on interpretation of the electrode positions. Using the Leksell stereotactic system, coordinates for each trajectory are then adjusted in the frame and a lateral view fluoroscopic image is performed in each new position. Care is taken to assure that the central beam of radiation during fluoroscopy is centered in the middle of the implantation probe in order to avoid parallax errors. If the trajectory is aligned correctly, corresponding to the planned trajectory and passing along an avascular space, the implantation is then continued, with skull perforation, dura opening, placement of the guiding bolt (AdTech, Racine, WI; Integra, Plainsboro, NJ), and final insertion of the electrode under fluoroscopic guidance. If a vessel is recognized along the pathway during fluoroscopy, the guiding tube is manually moved a few millimeters until the next avascular space is recognized and implantation is then continued. The electrode insertion progress is observed under live fluoroscopic control in a frontal view to confirm the straight trajectory of each electrode. For additional guidance, a coronal MRI slice corresponding to the level of each electrode implantation is overlaid onto the fluoroscopic image.
A postimplantation DynaCT scan is performed while the patient is still anesthetized and positioned in the operating table. The reconstructed images are then fused with the MRI dataset using the previously described fusion software. The resulting merged datasets are displayed and reviewed in axial, sagittal, and coronal planes allowing verification of the correct placement of the electrodes (21).
Following the surgical electrode implantation, patients are transferred to the epilepsy monitoring unit (EMU). The duration of admission at the EMU varies from patient to patient and depends on several factors including number and quality of recorded ictal and interictal patterns. In the last 4 years, patients undergoing SEEG implantation at Cleveland Clinic stayed on average 7 days (range from 3 to 28 days). After obtaining the necessary information, electrodes are removed in the operating room, in a procedure performed under local anesthesia and sedation. The results of the SEEG evaluation are discussed during another patient management conference, and recommendations for surgical resection are made. Patients are discharged the next morning, and resective surgery is scheduled 2 to 3 months following SEEG electrode removal (Fig. 81.2).
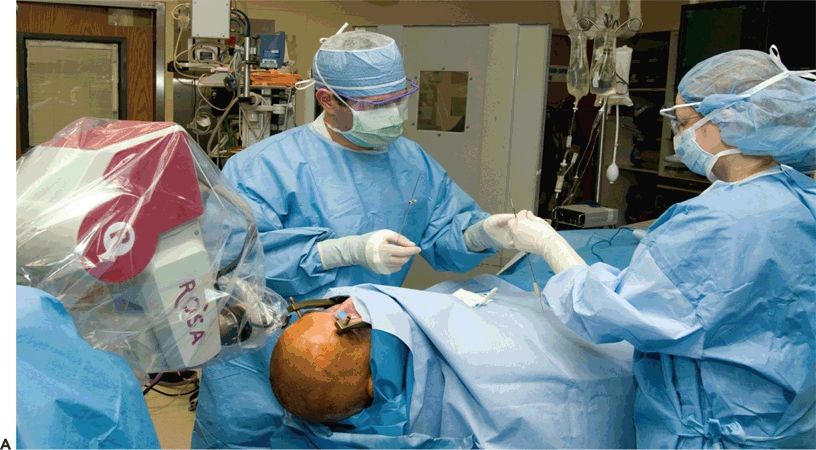
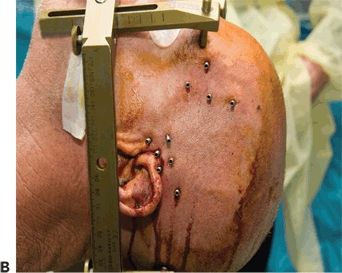
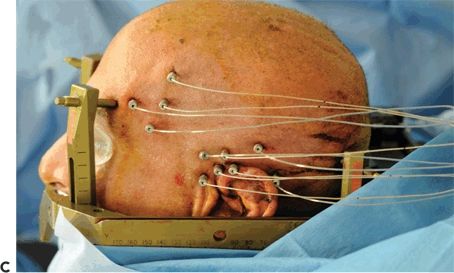
Figure 81.2. SEEG technique of implantation. A: General aspect of robotic implantation of SEEG electrodes, with the patient in supine position with the stereotactic robotic arm guiding the electrode implantation. B: Intraoperative picture, showing bolts implanted in stereotactic fashion, without the placement of electrodes. C: Final aspect of SEEG implantation, with electrodes placed in their final position.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








