Figure 24.1. The different compartments of TLE. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2014. All Rights Reserved.)
The pathology of nonmesial TLE subtypes runs through the usual categories, including tumors, vascular malformations, and encephalomalacia secondary to stroke and head trauma. But in medically refractory patients who had epilepsy surgery, the most important pathology is that of focal cortical dysplasia (29). As is discussed next, hippocampal sclerosis can coexist with another distinct pathology. The corresponding findings on imaging may be variable depending on the pathology. Many of the patients with nonmesial TLE who do not have an obvious lesion are called “MRI negative.” PET scans can show temporal hypometabolism in these “MRI-negative” cases, and pathology of resection specimens frequently reveals subtle focal cortical dysplasia.
These subcompartments of TLE lead naturally to the “Temporal Plus” concept. In a series of 80 patients (30) investigated by stereo-EEG (SEEG), 22 (27.5%) were classified into the Temporal Plus category based on the topography of initial EEG seizure onset, of which 9 involved the inferior frontal cortex, 7 the suprasylvian opercular cortex, and 6 the temporoparietooccipital junction. Hippocampal sclerosis by MRI was found in 77% of these patients, and they were treated surgically by resection of the temporal lobe and the additional cortical areas identified. When contrasted with patients with TLE proper, differences are found in auras, seizure manifestations, and scalp EEG along the lines expected based on the nonmesial TLE subtypes discussed. What remains far from agreement is the frequency of occurrence of Temporal Plus. The Grenoble cohort gave a high estimate of 27.5% in suspected TLE. By contrast, in the first 100 SEEG implantations into all lobes at Cleveland Clinic (31), only 5% had simultaneous frontal and temporal onsets treated by combined resection of the orbital or inferior frontal cortex and temporal lobectomy. There were no cases of temporoinsular epilepsy, but there were examples of frontoinsular or operculoinsular epilepsy. The biologic or pathologic basis of “Temporal Plus” can only be speculated upon. When there is a combination of hippocampal sclerosis and epileptogenicity beyond the temporal lobe, the situation can be viewed as an example of dual pathology. In this setting, pathologies such as focal cortical dysplasia or traumatic encephalomalacia are not necessarily bound to anatomical lobar boundaries.
DUAL PATHOLOGY IN TEMPORAL LOBE EPILEPSY
The term has been used in different ways, leading to possible misconceptions if definitions are not stated at outset. Experimentally, it has been applied to the creation of a two “hit” model of epilepsy, for instance, first irradiating rodent pups in utero who are then subjected to a second insult in the postnatal period. From the pathologist’s point of view, it means two separate definable pathologies. In practice, this usually means the presence of either hippocampal sclerosis or cortical dysplasia with another defined pathology (17,29). It is well known that cortical dysplasia is often associated with or contiguous to a neurodevelopmental tumor such as ganglioglioma and dysembryoplastic neuroepithelial tumor, wherever its location. Cortical dysplasia can present with hippocampal sclerosis in the same temporal lobe. The coexistence of focal cortical dysplasia with another pathology, be it tumor, hippocampal sclerosis, vascular malformation, or other, is now classified as FCD type III (29). Finally, dual pathology is also used to indicate the presence of two macroscopic and topographically separate structural abnormality detected by MRI.
Based on pathologic examinations, the presence of both hippocampal sclerosis and surrounding cortical dysplasia or other definition of malformation of cortical development occurs in 35% to 45% of temporal lobe resections (32,33). Others have defined one pathology, that of hippocampal sclerosis by imaging, and the other of cortical dysplasia by pathology in the resected specimen (34) and found that both coexist in 38%. Controversy abounds on what constitutes a true marker of a significant developmental abnormality in imaging studies of TLE. In one series of 30 patients with MRI-visualized temporal lobe developmental malformation based on blurring or signal change (but only 4 confirmed by pathology), 65% had associated hippocampal sclerosis based on MRI visual analysis and 87% by volumetric analysis (35). However, the histopathologic correlation of gray–white blurring and white matter signal increase in the anterior temporal lobe is a matter of controversy. One study found increased heterotopic neurons in the white matter (36). Others could not confirm these findings but identified a correlation of anterior temporal white matter blurring on MRI with myelin loss, increased water content, and axon degeneration (37,38), which may serve as markers of more prolonged or severe disease.
When dual pathology is defined by macroscopic findings on MRI, the incidence appears to be about 15%. In 100 patients with MRI volumetric or histologic evidence of hippocampal sclerosis, malformations of cortical development were found in 15% (39). But of interest, many of the malformations were either bilateral or on the side contralateral to the diseased hippocampus. Separately, in 167 patients with macroscopic lesions, whether temporal or extratemporal, 15% had an atrophic hippocampus defined by abnormal volumetric ratio (40). Atrophy is more common in patients with porencephalic cyst (31%), malformation of cortical development (25%), and gliosis (23.5%) as compared to tumors (2%) or vascular malformations (9%). Interestingly, the side of hippocampal atrophy can be found opposite to the side of the lesion. A history of febrile seizures occurred more frequently in those with hippocampal atrophy.
The clinical questions relevant to dual pathology include how each contributes to the epileptogenic process, whether one is the cause and the other the effect, and if resection of both is required for lasting seizure control in those considered for epilepsy surgery. Cortical dysplasia next to a neurodevelopmental tumor is frequently epileptogenic or at least potentially epileptogenic. Thus, invasive EEG recordings typically show seizure onset from an area next to the tumor, and a lesionectomy without removal of the surrounding epileptogenic areas more often results in surgical failure to control seizures. When cortical dysplasia occurs with hippocampal sclerosis in the same temporal lobe, there are more seizure onsets from the neocortical structures, either simultaneous with the hippocampus or independently as studied by combined subdural and depth electrodes (41). For this type of dual pathology with hippocampal sclerosis and histologic cortical dysplasia in the same temporal lobe, there is no major difference in postsurgical seizure outcome as compared to patients with focal cortical dysplasia alone, at least when followed over a 2-year period (42,43), although longer-term follow-up is necessary to learn if this pathology predisposes to late recurrences. When dual pathology is defined by two macroscopic lesions, it makes sense to remove both if they are amenable to safe resection within the same temporal lobe. The principle of removing both lesions becomes less certain when the distance separating the two gets bigger and bigger, as for instance, when there is hippocampal sclerosis in the temporal lobe and a separate lesion in the frontal, parietal, or occipital lobe (Fig. 24.2). Some authors recommend removal of both pathologies, but the experience is based on very small numbers (44). It becomes even more challenging when the lesions are in opposite hemispheres, or when hippocampal sclerosis coexists with diffuse cortical malformations in both hemispheres. For the foreseeable future, such cases will require invasive EEG evaluation to guide surgical planning on an individual basis.
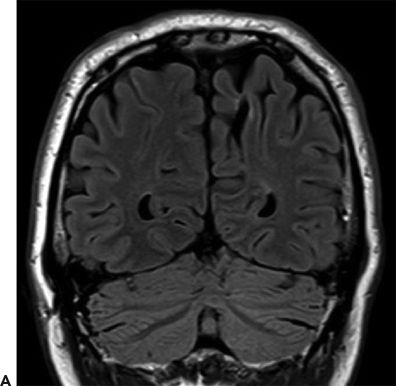
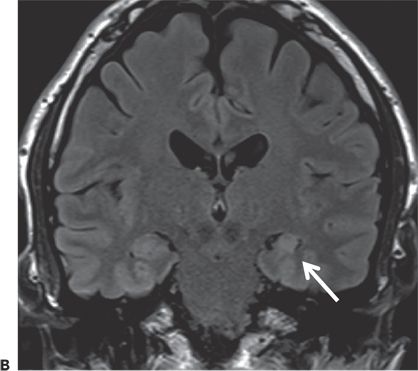
Figure 24.2. Case history: The patient had surgical correction of craniosynostosis as an infant. Seizure onset was at the age of 40 years. Early on, he had an aura of “being on the outside looking in,” but later, his seizure semiology changed and the aura presented with light-headedness and dizziness. Seizures evolved into chewing and hand automatisms, with right facial tonic deviation, followed by progression into a generalized convulsion. Scalp EEG showed left (60%) and right (40%) temporal sharp waves, with scalp ictal EEG patterns lateralized to the left hemisphere. SEEG investigation showed seizures arising from the left hippocampus and entorhinal cortex. He underwent left anterior temporal lobectomy and became seizure free. A: Left mesial parietal encephalomalacia. B: Left hippocampal atrophy and sclerosis (arrow).
ATYPICAL MANIFESTATIONS OF TEMPORAL LOBE EPILEPSY AND PSEUDOTEMPORAL EPILEPSY
The picture of TLE in childhood can be different, particularly due to modification by maturational factors. In the first few years of life, a child is unlikely to report auras but is likely to show behaviors in response to the experience, for instance by running to a parent for help. Early on, motor features can be rather nondescript with bilateral tonic or clonic limb movements or head nods (45). Seizures frequently manifest clinically with behavioral arrest and posturing or by a paucity of movements, a hypomotor seizure (46).
Other atypical manifestation include the coexistence of focal and generalized EEG discharges and seizures, exclusively generalized EEG discharge and seizures, and TLE looking like extratemporal epilepsy. In the latter, having an appreciation of the subcompartments of TLE and how seizures spread outside of the temporal lobe to other networks may help explain the extratemporal features. The coexistence of focal and generalized EEG features in patients with TLE is well known but the incidence unclear. In 101 patients who became seizure free after temporal resection (10), 38% had bilaterally synchronous spike–wave complexes as an interictal finding. This is surprisingly high as compared to 1% seen in the 67 TLE patients who were rendered seizure free after resection at a different center (15). Hypotheses to explain the coexistence of focal and generalized EEG features include an inherited trait of generalized epilepsy and other unknown synchronizing mechanisms producing bilateral generalized discharges, potentially in the setting of early developmental lesions. Less than 1% of patients truly express both clinical and EEG features of focal and generalized epilepsy, either chronologically with generalized seizures presenting earlier or concurrently (47). Even less frequent, generalized EEG discharges and seizures can be the only expression of surgically treated TLE (Fig. 24.3).
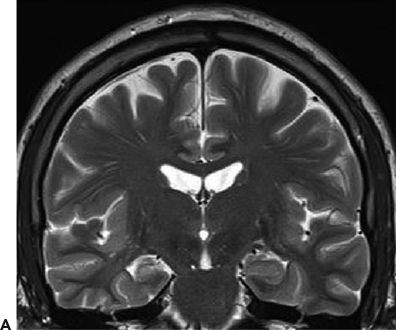
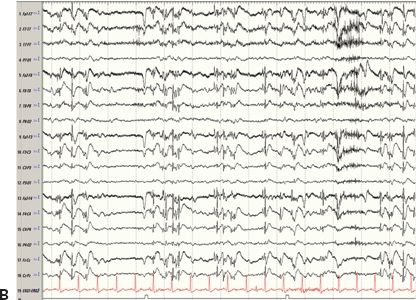
Figure 24.3. Case history: A 44-year-old man had febrile seizures twice at 2 years of age followed by afebrile seizures. Clinically, he had an aura of dizziness, and then either stared or entered a generalized tonic–clonic seizure. PET showed right temporal hypometabolism. Ictal SPECT (SISCOM) showed right temporal hyperperfusion. He has been seizure free after a right temporal lobectomy. A: Right hippocampal atrophy. B: Ictal EEG 6 minutes after initial onset. One minute later, the patient became unresponsive and entered a generalized tonic–clonic seizure.
It is also possible to mistake extratemporal epilepsy for TLE as encompassed by the term “pseudotemporal” epilepsy (48). The error is most commonly committed after a too cursory analysis of clinical semiology and scalp EEG findings. The eventual clues revealing seizures to arise from outside of the temporal lobe usually derive from the symptoms of the initial aura and imaging findings. Here, the temporal lobe acts as a “sink” to a propagated source, making the interictal and ictal EEGs, and part of the seizure evolution indistinguishable from that of TLE proper (49).
UNUSUAL CAUSES OF TEMPORAL LOBE EPILEPSY
A rare cause of TLE is an encephalocele or encephaloceles on the floor of the temporal fossa. They are most commonly found at the temporal tip near the ridge of the sphenoid bone. In earlier case reports, the pathology was often not suspected and only uncovered during surgery (50). The clinical and EEG manifestations are indistinguishable from mesial TLE, and therefore, these findings were previously regarded as MRI-negative TLE. But improvements in MR and CT imaging now nearly always permit preoperative diagnosis as long as the studies are done and looked at properly (51). Imaging reveals erosion of the greater wing of the sphenoid bone and protrusion of either CSF or brain signal material below the temporal floor (Fig. 24.4). Single or multiple adjacent encephaloceles are seen at operation. Pathology reveals a mix of gliosis, disorganization, or dysplasia in the prolapsed part of the cortex and sometimes with similar pathology in the amygdala. This is a surgically remediable pathology of TLE.
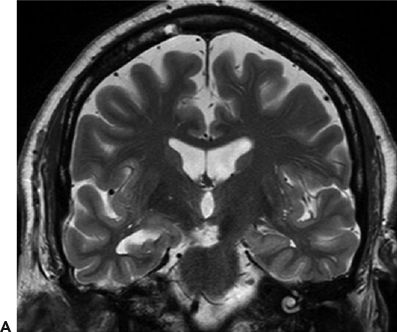
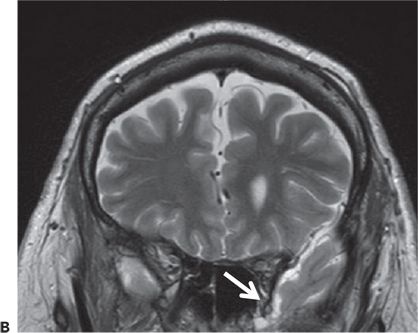
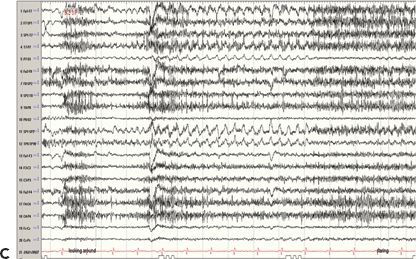
Figure 24.4. Case history: This is a 71-year-old man with seizures since the age of 48 years. Seizures occur without aura or warning. He either stares off or enters a generalized tonic–clonic seizure. Cursory examination of the MRI raised question of right hippocampal atrophy. Interictal sharp waves are left (70%) and left (30%) anterior and mesial temporal while three seizures recorded showed a left temporal-onset pattern. PET study confirmed left temporal hypometabolism. A: Right hippocampal atrophy. B: Left anterior temporal encephalocele (white arrow). C: Ictal EEG onset in form of rhythmic 3-Hz sharp waves from left anterior temporal region.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








