Chapter 35 The Human Circadian Timing System and Sleep–Wake Regulation
Abstract
The circadian pacemaker (or biological clock) is phylogenetically ubiquitous, found in species from prokaryotes to humans. Circadian pacemakers have several defining characteristics: endogenous rhythmicity that persists independent of periodic changes in the external environment, a near-24-hour period (circadian from Latin circa meaning “about” and dies meaning “day”), and the capacity for environmental input to modify or reset timing or phase of the rhythm.1–3 We provide an overview of the human circadian timing system, including the pacemaker with its inputs and outputs, and describe how this pacemaker interacts with sleep–wake regulatory processes to influence physiologic variables including hormone levels, autonomic nervous system activity, neurobehavioral performance, and the propensity for and timing and internal structure of sleep. We also consider the influence of episodic and daily recurring behaviors, including sleep itself, on these physiologic variables relative to that of the endogenous circadian pacemaker.
Identifying the Mammalian Circadian Pacemaker
In mammals, the suprachiasmatic nucleus (SCN) in the anterior hypothalamus is the central neural pacemaker of the circadian timing system. On the basis of careful patient histories characterized by disruptions of sleep–wake timing (e.g., insomnia, reversal of the sleep–wake schedule), Fulton and Bailey4 postulated in 1929 a region in the anterior hypothalamus that appeared to regulate not the occurrence of sleep but its timing within the 24-hour day. It was not until in 1972 that the SCN was identified as the site of the mammalian circadian pacemaker,5,6 and further research has conclusively established the SCN as a principal source of endogenous rhythmicity in mammals.7 Physiologic studies show that multiple distributed circadian oscillators drive daily rhythms in peripheral systems.8 Molecular research confirms the presence of peripheral clocks that use the same molecular machinery as the central circadian pacemaker in the SCN. Pacemakers like the SCN convey internal synchrony to these distributed oscillators.
Influence of Sleep and Circadian Rhythms on Human Physiology
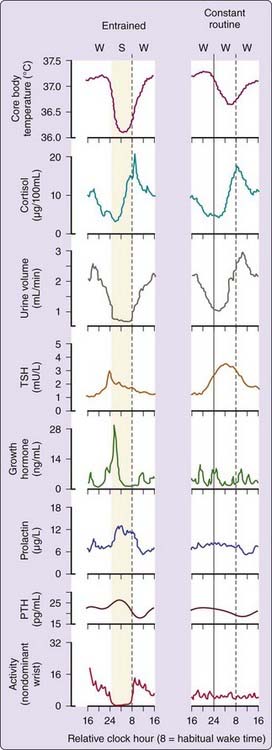
The discovery of the SCN’s role as a central circadian pacemaker set the stage for understanding how it drives the prominent daily fluctuations in a wide array of physiologic functions in human subjects synchronized to the 24-hour day and on a normal sleep–wake schedule, (Fig. 35-1, left column of panels).9–17 Core body temperature is lowest and melatonin levels (not shown) are highest11,16,18 during night sleep. Cortisol is low at habitual sleep onset but high at habitual morning wake time. The temporal profile of each of these parameters when these endogenous circadian rhythms are entrained or synchronized to the 24-hour day exhibits a characteristic fingerprint that results from a combination of drives from the timing of the sleep–wake state to the endogenous circadian pacemaker and responses evoked by other factors such as posture, mood, exercise, and environmental lighting.16,19
To characterize the circadian pacemaker–driven component of a diurnal temporal profile from the effects of sleep–wake state, behavior, posture, and periodic and environmental stimuli, the constant-routine protocol originally proposed by Mills and colleagues20 has been refined and extended.21 In the constant routine, subjects typically undergo continuous enforced wakefulness throughout day and night in a constant posture at a constant minimal activity and in constant, relatively dim, ambient illumination.22 Under such conditions, the temporal profiles of many physiologic variables are significantly altered, and we can separate the component of these rhythms that is driven by the endogenous circadian pacemaker from those that reflect changes in the sleep–wake state, posture, or periodic external environment.21 Given the influence of posture23 and the minimal influence of sleep24 on the endogenous circadian melatonin rhythm, we have sometimes used a constant posture protocol in which subjects are maintained in a constant semirecumbent posture in constant dim light but are allowed to sleep at night so that melatonin circadian phase can be assessed.
Body temperature declines during sleep19,25–27 as illustrated by the profile of core body temperature recorded during a normal sleep–wake schedule and on a constant routine (see Fig. 35-1, right column of panels). It is apparent that the sleep episode itself (including associated changes in posture, light intensity, and activity level) generates a drop in body temperature relative to wake.8,19,27–31 This sleep episode–induced drop in temperature combines with the circadian-driven decline in body temperature (which exhibits a nadir during the latter half of the habitual nocturnal sleep episode) to yield a larger apparent amplitude than that of the endogenous circadian component alone (as measured on constant routine). Urine volume is another variable that exhibits a robust oscillation under constant-routine conditions but is also influenced by sleep–wake state.11,32
Rhythmicity in some variables appears nearly independent of sleep–wake state. The temporal pattern of the hormone melatonin is relatively unchanged whether a subject is asleep or awake all night on a constant routine,33 although significant age-dependent effects of sleep and sleep deprivation on melatonin amplitude have been quantified.24 Posture is reported to influence circulating melatonin concentrations somewhat.23 The temporal profile of cortisol is also relatively unchanged whether a person sleeps on a habitual schedule or remains awake all night, although cortisol levels are elevated on the following afternoon and evening.34 Plasma cortisol concentrations can be suppressed if sleep onset occurs at the crest of the cortisol rhythm rather than at the nadir.35
Several other hormones are sensitive to sleep–wake state. Sleep opposes the circadian rhythm regulating thyroid-stimulating hormone (TSH), inhibiting TSH release during the peak of the endogenous circadian TSH rhythm, which otherwise occurs in the middle of the night.12,15,36–38 Under entrained conditions, nocturnal TSH is blunted by the timing of sleep such that TSH levels are highest just before sleep onset and continue to be suppressed during the remainder of the sleep episode. This inhibitory effect of sleep on TSH secretion has been closely associated with slow-wave sleep39 and with relative delta power in the sleep electroencephalogram (EEG).40 Growth hormone, prolactin, and parathyroid hormone levels all show a prominent sleep-dependent increase.37,41 For growth hormone, a major sleep-related secretory episode is associated with slow-wave sleep42 and with relative delta power of the sleep EEG,43 though such associations for prolactin are controversial.44–46 Interestingly, growth hormone levels blunted by acute sleep deprivation are elevated by recovery sleep such that the average 24-hour levels are conserved,47 and following sleep restriction for 1 week, they are significantly elevated by the combination of a presleep and circadian-related secretory episode and typical but prolonged sleep-related response.47
Leptin levels exhibit circadian rhythmicity, although the typical day–night pattern is reflected in the interaction of circadian rhythmicity with energy intake and expenditure17 and sleep duration.48 Ghrelin levels exhibit a day–night variation related to energy intake related to the presence of sleep49 and to sleep duration.50
Ultradian variations in the release of renin from the kidney—a key factor in blood pressure control—are closely linked to the timing of the rapid eye movement (REM) and non-REM (NREM) sleep cycle,51,52 an association evident even among patients with disturbed sleep, whose plasma renin profiles reflect pathologic changes in sleep structure. Increased relative delta power in the sleep EEG is associated with increased levels of plasma renin activity, whereas decreased slow-wave activity is associated with a decrease.53
In 1979, Aschoff pointed to some evidence for an endogenously rhythmic component even among sleep-dependent hormones, particularly with respect to the magnitude of the response evoked by sleep.54 Using the constant-routine protocol, it has been shown that in the absence of sleep, prolactin and parathyroid hormone also have an endogenous circadian component that is lowest a few hours after habitual wakeup time.13,14,37 A study involving repeated injections of growth hormone–releasing hormone (GHRH) across the day demonstrated elevated peak growth hormone (GH) responses in the evening, suggesting that diurnal variation in GH secretion reflects, in part, circadian rhythmicity in the response to GNRH.55
Effects of the interaction between sleep-dependent and circadian factors on these hormones can be important when the sleep–wake schedule is not synchronized with the circadian pacemaker. With shift workers who must acutely remain awake on the first night shift, for example, one would expect a substantial increase in TSH release over secretion under normal sleep–wake conditions during entrainment (see Fig. 35-1). On the other hand, these workers would be deprived of their normally higher levels of growth hormone, prolactin, and parathyroid hormone during the waking night. Such alterations of the profiles of a variety of hormones have been documented in laboratory studies of night-shift workers.56 In a laboratory simulation of circadian misalignment with the imposed sleep–wake and eating schedule induced by night shift work and transmeridian travel, subjects exhibited increased leptin and increased glucose (despite increased insulin) and misalignment of the endogenous circadian rhythm of cortisol secretion with respect to the inverted sleep–wake schedule, along with the expected reduction in sleep efficiency.57
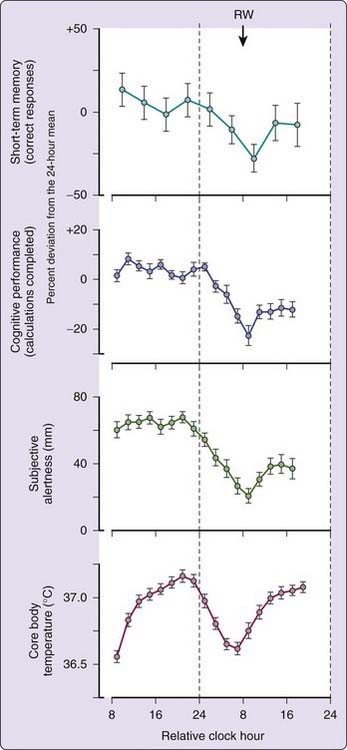
The circadian pacemaker significantly influences a variety of neurobehavioral and cognitive functions.58–64 Under the conditions of the constant routine, subjects display a circadian variation in short-term memory, cognitive performance, and alertness that is tightly coupled to the timing of the body temperature rhythm (Fig. 35-2).65 During a constant routine, these cognitive functions tend to be at their nadir shortly after habitual wakeup time due to an interaction between sleep loss and the circadian rhythms of performance.
Effects of Light on Human Circadian Rhythms
The light–dark cycle is the primary environmental signal that synchronizes circadian systems in a wide array of species, including humans.1,11,19,22,32,33,66–68 Nonvisual, or non–image-forming retinal photoreception provides input to the circadian system, the pupillary light reflex, and other systems. Direct retinal input travels via the retinohypothalamic tract, a monosynaptic pathway by which information about the environmental light–dark cycle reaches the SCN.69–74 Postmortem studies reveal that the human brain contains the same key structural elements—the SCN and retinohypothalamic tract—as that of other mammals.71,75,76 Neuropathologic studies associate damage to these structures with abnormalities in the timing of the sleep–wake cycle and other circadian rhythms.77–79
More recent research in rodents and humans shows that the three-cone system and rods, the visual photoreceptors, are not required for transmitting light signals to the circadian system.72–74,80–84 Retinal ganglion cells were thought only to pass on information from the rods and cones, but a distinct set of ganglion cells in the inner retinal layer that project to the SCN70 are intrinsically photosensitive. Only the ganglion cells that project from retina to SCN selectively contain the vitamin A–based photopigment melanopsin.72,85 Animal studies determining the action spectrum show that blue-green light (about 450 to 500 nm), which matches the sensitivity peak of melanopsin, is the most potent in shifting circadian phase in animals83,86 and for melatonin suppression in humans,80,81,84 consistent with data on phase-shifting responses in humans.84,87 Within this specific set of intrinsically photosensitive retinal ganglion cells, melanopsin is the active photopigment. Rods and cones that synapse onto melanopsin-containing ganglion cells also participate, creating redundancy in circadian photoreception.88,89
A neural output pathway of the SCN passes through the intermediolateral cell column of the upper thoracic spinal cord, to the superior cervical ganglion which provides sympathetic input into the pineal gland.90 The absence of melatonin in patients who have cervical spinal cord injury is due to disruptions of this neural pathway to the pineal gland91 and is associated with decreased sleep efficiency.92
Photic Suppression of Melatonin Secretion
The neural pathway from the SCN to the pineal provides for the regulation of the pineal output of melatonin by the SCN,90,93 including inhibition of melatonin release by retinal light exposure via a retinolypothalamic pathway,94 which can be used as an assay for the functional input of light into the circadian system.95,96
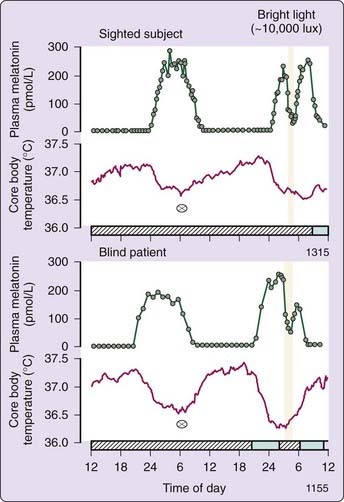
Preservation of light-induced melatonin suppression in otherwise totally blind people, suffering from severe damage to the outer retina,96 led to the discovery that a distinct visual system mediates photic entrainment.97,98 The nocturnal increase in melatonin is illustrated in Figure 35-3 (upper panel) for a normally sighted subject on a constant routine.96 During a second peak of melatonin on the next night, a bright light stimulus induced an acute suppression of melatonin levels, which returned to elevated nighttime levels after light exposure was terminated. In the lower panel, bright light still suppresses melatonin even in a totally blind subject with no conscious light perception and a negative electroretinogram. The loss of conscious light perception does not necessarily indicate the loss of photic input to the circadian timing system,96 although this was not the case in all such blind individuals. Two distinct visual systems exist: one for visual perception and a separate non-image forming visual system for synchronization of the circadian pacemaker in the SCN, alerting input to the sleep switch in the ventrolateral preoptic area (VLPO), suppression of melatonin secretion, and mediation of the pupillary light reflex.70–89,96–98
Human Phase-Response Curves to Light
In circadian biology, the phase-response curve (PRC) is used to characterize the synchronizing effects of light on a circadian pacemaker.1,22,99,100 To construct a photic PRC, discrete light stimuli are applied systematically over the entire circadian cycle, and the magnitudes of light-induced phase shifts are plotted as a function of circadian phase at which the organism is exposed to the stimuli. In human experiments, the constant routine has been used to estimate both the initial circadian phase of the pacemaker before stimulus and the final circadian phase after stimulus. The difference between the initial and final circadian phases represents the light-induced phase shift.
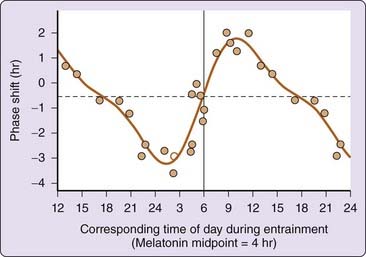
All circadian systems exhibit a characteristic photic PRC, in which the largest light-induced phase shifts are generated in the subjective night of the circadian cycle. Phase delays are generated to light stimuli in the late subjective day and early subjective night, and phase advances are generated in the late subjective night and early subjective day.1 Figure 35-4 illustrates the PRC to a single pulse of light in humans. In humans, phase delays were observed in response to single, 6.7-hour bright light pulses applied before the minimum of the core body temperature cycle, on average about 2.3 hours before habitual wakeup time. Phase advances were observed when such light pulses were applied after the core body temperature nadir. The resultant human PRC to single light pulses101 exhibits the classic pattern of light PRCs in many organisms,1,100 including phase advances and delays.
The response to light during the subjective day of a three-pulse PRC reveals that the human circadian pacemaker responds to light throughout the subjective day,102 a characteristic of the human PRC that is similar to that reported for other species,1,100 and suggests that appropriate light intensities can shift the phase of the human pacemaker in morning and late afternoon or evening as well as at night. This has important clinical implications, such as use of phototherapy to reset circadian phase in delayed or advanced sleep phase disorder.
Photic Resetting of the Pineal Melatonin Rhythm
Because circadian rhythms are expressed in many physiologic and neurobehavioral variables, the phase of the pacemaker may be estimated by using any of these variables as a marker. In humans, the core body temperature rhythm is often a preferred marker of circadian phase, because it can accurately represent the underlying pacemaker’s characteristics under certain conditions. However, melatonin can be an even more precise circadian marker,11,33,103,104 which is less heavily influenced by sleep and posture.23,105 In humans studied during a constant routine, melatonin reflects the phase of the underlying pacemaker following light-induced phase shifts better (less variability) than the endogenous component of the core body temperature rhythm.105 Both rhythms shift equivalently whether to an earlier or a later hour.11,104 Such studies demonstrate that the endogenous circadian melatonin rhythm can be reset to any desired phase within 2 to 3 days by light exposure.104 Furthermore, photic stimuli designed to suppress the amplitude of the endogenous circadian temperature cycle also suppress the amplitude of the endogenous circadian melatonin rhythm.104
The use of the melatonin rhythm as a circadian marker has additional practical advantages: melatonin in human saliva correlates well with that in plasma, and it allows the evaluation of circadian phase in patients with suspected circadian rhythm disorders or research subjects relatively noninvasively.106
Human Dose-Response Curve to Circadian Phase-Resetting Effects of Light
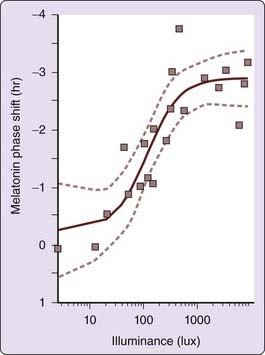
In addition to dependence on wavelength, the degree of light-induced phase shift also depends upon light stimulus intensity and consecutive days of exposure. Three consecutive daily pulses of light can generate a larger phase shift than a single light pulse. This intensity relationship also applies to brightness or illuminance level of light to which the retina is exposed. Following a 6.5-hour, single bright light stimulus of different intensities at a circadian phase known to generate a phase delay, an increase in resetting response is seen at 50 lux, with maximum slope at 100 lux and maximum shifts by about 550 lux. The light intensity versus resetting response relationship is clearly nonlinear (Fig. 35-5)107 and can be predicted with a mathematical model.108,109
The observation that ordinary room lighting of ~100 lux with only 1% of the intensity induces 50% of the resetting response to a 10,000 lux stimulus has important implications. We are exposed to bright light for a relatively short time each day,110–112 but in modern industrialized societies we are exposed to ordinary indoor room light for many hours, a predominance of exposure that may have a greater impact on our circadian system than a few minutes of exposure to bright light.
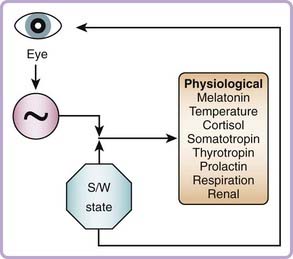
Figure 35-6 illustrates the influence of the circadian pacemaker and the sleep–wake state on physiologic variables and the influence of light input via the eye to the circadian pacemaker. The feedback loop from the sleep–wake state to the eye represents the effects of exposure to the environmental light cycle, because the sleeping state in humans is usually associated with eyelid closure and self-selected exposure to darkness, achieved by drawing window shades and switching off artificial light sources, whereas the waking state in humans is usually associated with opening of the eyelids and exposing the retina to light via self-selected use of artificial light or exposure to outdoor light during waking hours. Under a strict sleep–wake and light exposure schedule, the pacemaker’s timing is consistent from day to day. However, whenever sleep is initiated late or terminated early, or a waking episode occurs within a sleep episode, the associated light exposure can reset the pacemaker. This association between waking and light exposure and the fact that low light intensity has a significant resetting effect on the pacemaker has practical relevance for routine sleep–wake scheduling and for understanding the influence of sleep disruption, which is often associated with light exposure, on circadian phase.
Nonphotic Circadian Phase Resetting and Reentrainment
Nonphotic input to the human circadian system is less well characterized than photic input. Results of early studies, which focused on social cues such as gong sounds or regularly scheduled performance tests, meals, and bedtimes,113 were confounded by limitations of phase measures used and self-selected lighting conditions. Exposure of healthy young men to nocturnal exercise of 1 to 3 hours’ duration resulted in phase delays in nocturnal melatonin the following day114,115 that appeared more consistent after a 3-hour moderate-intensity session than after a 1-hour high-intensity session.116 Early evening 1-hour high-intensity exercise (at approximately 6:30 PM) elicited phase advances significantly different from the phase delays in response to morning, afternoon, and nocturnal exercise and in no-exercise subjects (Fig. 35-7). In a study of the facilitation of re-entrainment to a delay-shifted sleep–wake episode in extremely dim light (to control for ambient light during exercise), exercise during the biological night produced phase delays compared with no exercise.115 Thus, appropriately timed nonphotic stimuli such as exercise or other forms of arousal can facilitate adaptation to acute changes in light–dark cycle.
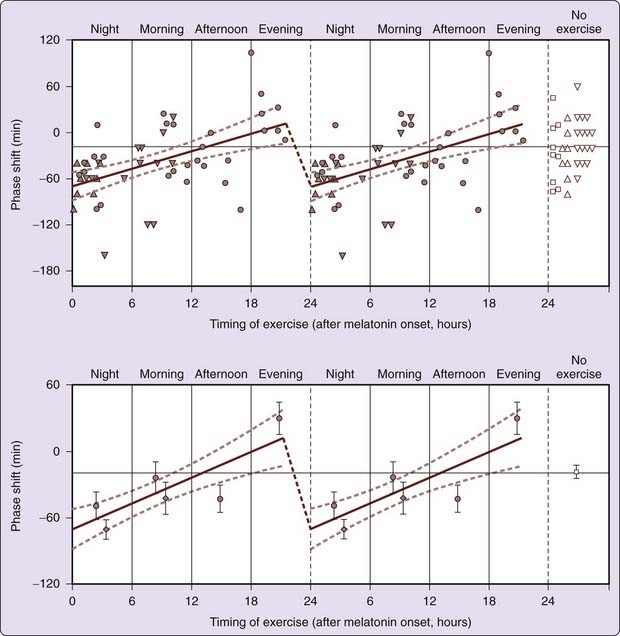
Figure 35-7 Phase-response curves in response to exercise at different circadian times of day. Phase delays were observed in response to nocturnal exercise, phase advances were observed in response to exercise during late afternoon or early evening. Closed circles indicate phase shifts in response to high-intensity, 1-hour nocturnal exercise and daytime exercise. Upward165 and downward triangles166 (top panel) and diamonds (bottom panel) indicate phase shifts in response to low-intensity, 3-hour exercise sessions. The line indicates a significant relationship between phase shifts and circadian time of exercise (r2 = 0.28, P = .0003; slope significantly different from zero). Dashed curves indicate 95% confidence intervals of slope of line.
(Reproduced with permission from Buxton OM, Lee CW, L’Hermite-Balériaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin levels that vary with circadian phase. Am J Physiol 2003;284(3):R714-R724.
That appropriately timed exposure to exercise also results in phase advances is demonstrated by partial entrainment to a 23.5-hour light–dark and sleep–wake schedule in healthy volunteers exercising at moderate intensity twice daily (midday and late afternoon) over 2 weeks.117 Subjects exercising daily in late afternoon exhibited partial entrainment, advancing on average 10 minutes per day more than nonexercising controls, consistent with phase-advancing effects of late afternoon or early evening exercise on the human circadian clock. Given the slightly greater than 24-hour endogenous circadian period of humans and the net daily phase advance required for stable entrainment, evening exercise, particularly repeated daily exposure, could result in daily phase advances leading to nonphotic entrainment of the human circadian system if the timing and intensity of the exercise were optimized.
Investigating Circadian and Sleep–Wake Dependent Modulation
The Kleitman Protocol
Separation from 24-Hour Environmental and Behavioral Cues
Nathaniel Kleitman was the first investigator to study human circadian rhythms in the absence of periodic 24-hour cues in the external environment (Fig. 35-8).61 Core body temperature records from one of his two subjects in Mammoth Cave, Kentucky, in 1938, who underwent a 28-hour imposed sleep–wake schedule, were compared with laboratory data collected at the University of Chicago from the same subject living on a 24-hour routine61 (shown in Fig. 35-9). On a 24-hour schedule, there were seven cycles of the body temperature rhythm, as one would expect over the course of a 1-week recording. The week with an imposed 28-hour schedule also has 7 cycles of body temperature rhythm, but only 6 sleep–wake cycles (see Fig. 35-9, upper panel). Despite the confounding effect of sleep itself on core body temperature, this experimental protocol still separated the influence of timing of the sleep–wake schedule from that of the circadian pacemaker—at least in this subject.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree