In addition to this financial burden dementia exerts an enormous personal and emotional cost on the people affected, as well as their families. Caring for a person with dementia can be a distressing experience, and informal care, in which the families or close friends care for the person, accounts for a large proportion of people with dementia who live in the community. The impact on these informal carers is difficult to quantify, often affecting quality of life and well-being beyond the direct impact of the condition on the individual themselves.
SYMPTOMS AND PROGRESSION OF DEMENTIA
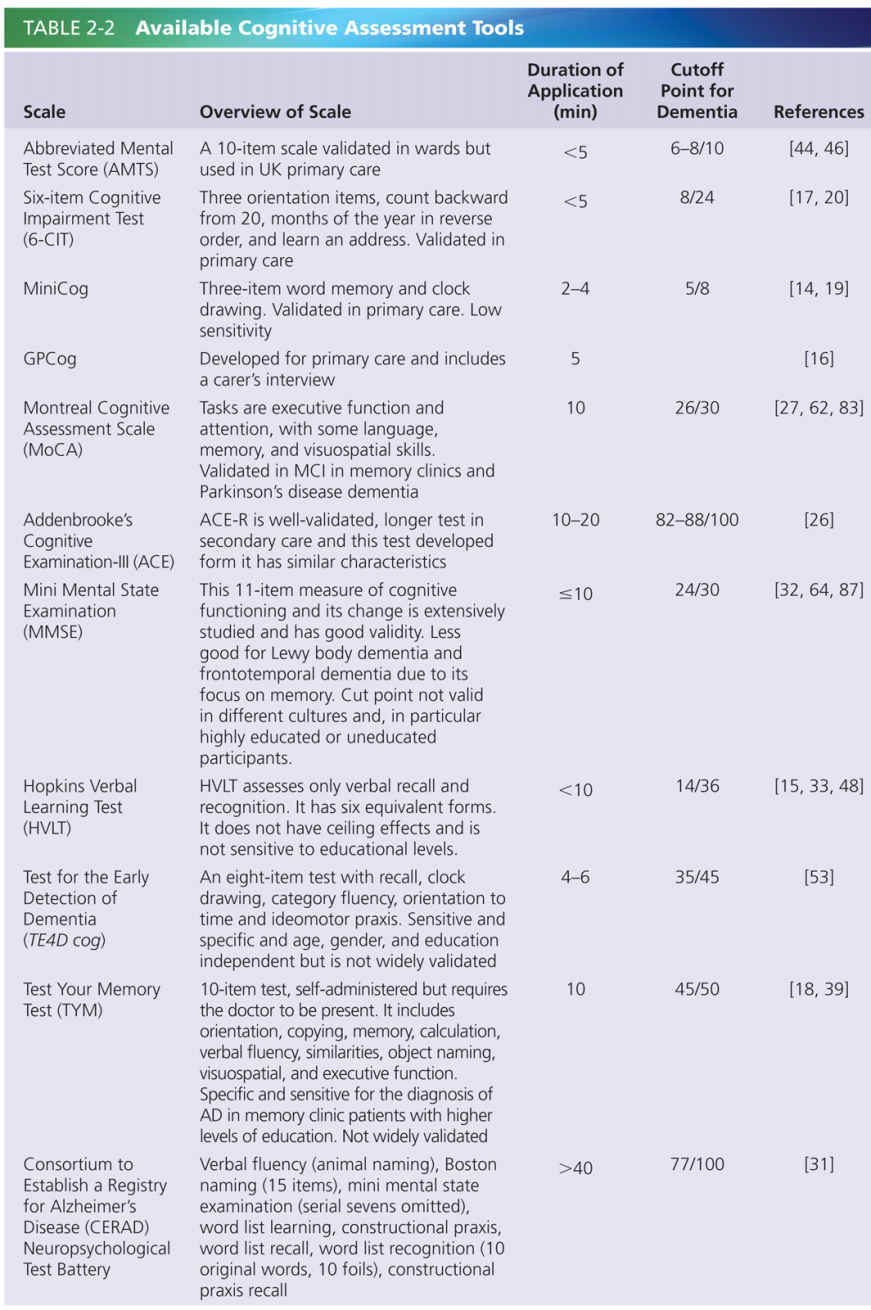
The symptoms experienced by a person with dementia are dictated by the area of the brain affected, and by the underlying cause of the condition. The broad functions of the brain and likely symptoms that would result if they were to be affected are shown in Fig 2-1.
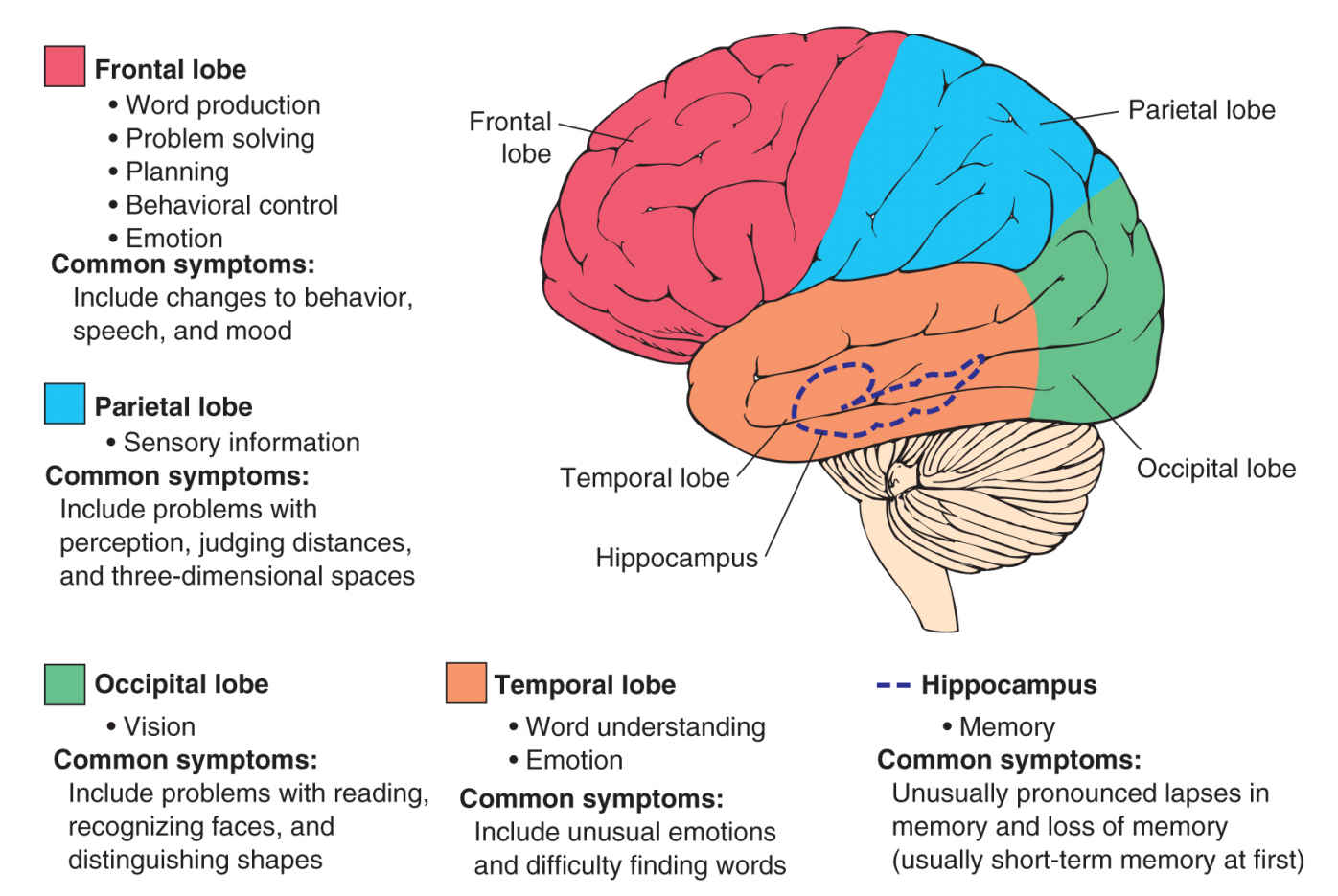
FIGURE 2-1 The challenge of concurrent pathology. One of the biggest challenges for biomarker research is the substantial overlap of major brain pathologies. For example,
• 90% of people with dementia with Lewy bodies have significant concurrent Alzheimer’s disease (AD) pathology.
• Almost all patients with significant cerebrovascular disease also have substantial amyloid pathology, particularly in people over the age of 80.
• 40% of patients with AD have significant cerebrovascular disease.
Early symptoms of cognitive and functional impairment that occurs in dementia may include, but are not limited to, the following:
• Memory loss or uncharacteristic lapses in memory, particularly in short-term memory. While memory loss is very commonly associated with the early signs of dementia, particularly Alzheimer’s disease (AD), this is not true for all patients, some of which show no memory dysfunction until much later in their condition.
• Difficulties in language and communication. Although a person was previously articulate, he or she may show a loss of vocabulary or difficulty in forming sentences.
• Changes to personality or behavior. This is particularly common in frontotemporal dementia (FTD) and may result in a misdiagnosis of depression.
• Difficulties in coordination and motor control. This symptom is often difficult to distinguish from Parkinson’s disease, which can also be associated with a form of dementia.
• Difficulties with vision, for example, in the ability to read or recognize faces and shapes
• Difficulties in performing everyday activities that were previously easy to manage, such as household accounts or shopping
The breadth and scope of symptoms is often a critical obstacle to achieving a diagnosis, and dementia is commonly misdiagnosed and remain undetected over long periods. Diagnosis of dementia is achieved initially through psychological testing in combination with consultation with the individual and a close family member or friend to identify signs of impairment. Many assessment tools are available for clinicians to detect cognitive impairment, most of which focus on assessing memory, reasoning, and processing as measures of cognition. Where there is an indication of cognitive impairment a diagnosis will be gained through further in-depth psychological testing. Different neuropsychological tests are available, and are recommended for use in specific settings. Based on the outcome of these tests dementia is usually categorized as mild, moderate, or severe, although the distinction between these stages is frequently blurred. Currently available, recommended tests are summarized in Table 2-2. Diagnosis of dementia may involve neuroimaging in an attempt to discover the underlying cause of the dementia. The use of computed tomography (CT) or structural magnetic resonance imaging (MRI) is recommended to identify key pathologies that may contribute to dementia, such as a tumor, hydrocephalus, or cerebrovascular disease, and to detect brain atrophy [91]. Loss of brain volume in the medial temporal lobe is a particular signature of dementia, and studies indicate that this hallmark may be detected in people with mild cognitive impairment (MCI) before they develop dementia [8]. In some cases diagnosis may also be enhanced through the sampling of cerebrospinal fluid (CSF) through a lumbar puncture to detect specific peptide biomarkers of causative diseases [73]. An accurate dementia diagnosis allows the detection of potentially treatable disorders, facilitating planning of future life and finances, including advance directives, and optimal treatment and care.
Progression of symptoms will similarly be dictated by the progressive underlying pathology that is present in each case of dementia. Cognitive impairment frequently affects one cognitive or functional domain in the early stages, spreading to more pervasive impairment over time. The speed and pattern of decline is often indicative of the underlying cause. For example, AD usually progresses through a steady decline, while vascular dementia (VaD) is characterized by sudden decline in stepwise increments. Neuropsychological testing and neuroimaging is also used to monitor the progression of dementia. This is essential as it informs decision-making about treatment choice and judgments relating to an individuals’ capacity, particularly in making decisions about their future care and treatment.
A common and distressing set of symptoms are behavioral and psychological symptoms of dementia (BPSD) such as agitation, aggression, psychosis (hallucinations and delusions), apathy, and depression. These symptoms are particularly frequent in people with frontotemporal dementia but will affect 90% of people with dementia at some point in their condition [24]. BPSD present a particular challenge for carers, and are often a trigger for people moving to a care home setting [34]. In addition to arising as a result of dementia pathologies, BPSD are frequently caused by underlying issues such as untreated pain and discomfort or environmental factors within the care setting. Early detection and management of BPSD is vital in order to address any causative factors and to inform treatment decisions.
TREATMENT OF DEMENTIA
Treatment of dementia relies on rapid clinical response following diagnosis and on effective communication with person and their families. Although there is no long-term effective treatment, early intervention provides the best opportunity to maximize a person’s cognition in the earlier stages of the condition. This is particularly valuable as it enables people to plan their future treatment and care while they have the capacity, and to take an active role in decision-making. However, few pharmacological options are available for treatment for people with dementia. Four medications, rivastigmine, donepezil, galanthamine, and memantine, are currently licensed for treatment of the symptoms of AD, but currently no licensed drugs are available for other types of dementia. People with dementia frequently have diagnoses of comorbidities such as cardiovascular disease, cancer, and infections. Therefore, the potential cognitive impact of concomitant medications should be considered. Nonpharmacological treatments such as cognitive training, stimulation, and rehabilitation may be helpful in reducing cognitive decline [96].
A particular treatment challenge for clinicians is the management of BPSD. In the absence of effective alternatives, these symptoms have frequently been treated with antipsychotic medications despite the very limited license available for this practice. Evidence to support the use of the antipsychotic risperidone for short-time periods up to 6 weeks is available, but very little benefit is seen with other antipsychotics or with long-term prescriptions [7]. Furthermore, antipsychotics are associated with considerable severe side effects, which include worsening of cognitive decline, falls, sedation, stroke, and increased mortality. Following numerous initiatives to reduce antipsychotics in people with dementia there has been a substantial drop in their use. However, a culture of antipsychotic prescribing continues to exist despite guidance for their judicious use. This issue is exacerbated by the lack of effective pharmacological treatments. However, a growing body of evidence supports the use of nondrug psychosocial approaches to prevent and manage BPSD [24]. The most promising of these are embedded within the principles of person-centered care, the gold standard for dementia care which promoted a personalized, tailored approach to care. Studies have shown that simple structured approaches to provide personalized activities and social interaction can improve BPSD such as agitation and aggression [86]. These approaches are key elements in best practice for dementia care, although a lack of standardized training for health-care professionals is a barrier to their implementation.
CAUSES OF DEMENTIA
Over 100 conditions and diseases that can cause dementia through neurodegeneration exist. The majority affect older adults and relate to the development of specific pathologies in the brain, although rarer causes can occur in younger individuals as a result of specific events or lifestyle factors, for example, as a result of a brain injury or excessive alcohol consumption. In addition to the numerous established causes there is evidence of general risk factors for dementia which increase the likelihood of dementia later in life when considered on a population basis. These include midlife hypertension and hypercholesterolemia, diabetes, obesity, and stroke [8]. This evidence supports the view that from a public health perspective prevention of dementia could be achieved through management of risk factors in order to reduce the risk of development of underlying diseases and conditions that lead to dementia. The various causes of dementia are complicated by the considerable overlap in underlying pathologies, which result in a blurring of boundaries between different types. The most common causes of dementia are discussed in detail in the subsequent sections.
ALZHEIMER’S DISEASE
AD is the primary cause of dementia worldwide, affecting up to 80% of people with the condition [4]. AD is most common in people with dementia over 65 (late-onset AD), although AD can develop during midlife (early-onset AD), usually due to rare genetic factors. Cognitive decline in AD usually follows a steady trajectory, frequently commencing with impairment in memory as AD commonly develops in the hippocampus.
Pathology
The two core pathological hallmarks are amyloid plaques and neurofibrillary tangles that develop in the brain of a person with AD. This accumulation of proteins is central to the “amyloid cascade hypothesis,” in which amyloid-β peptides are thought to trigger neuronal dysfunction [61]. This in turn is thought to influence the processing of the tau protein, resulting in intracellular “tangles” that contribute to cell death. The sequence of pathological events and causal relationship of AD pathology remains unclear, and the precise relationship between amyloid-β and tau has not been established [82]. More recent evidence challenging the established hypothesis and suggesting that amyloid pathology is the result, rather than the cause, of dysfunction [11].
Genetic Risk Factors
A number of genes have been identified as playing a role in the development and risk of AD. These include dominant mutations of the genes encoding amyloid precursor protein (APP) and presenilins (PSEN1 and PSEN2) [35, 49, 79]. These genes account for only 5% of people with AD, and result in early-onset AD. These genes hold significant risk for AD, and usually manifest in a clear hereditary link.
In late-onset AD the genetic causes are less clear-cut. There are several risk genes, including ApoE4, which confers a sevenfold increased risk, although this translates to only a very small overall increased risk, particularly when viewed from a population perspective [25]. More recently, research has identified additional genes such as TOMM40, CLU, and PICALM, but large cohort studies are needed to confirm their role [42, 78, 81]. The gene SORL1 has been highlighted as an important genetic cause of late-onset AD, and at least one further familial AD gene has not yet been identified [12, 74].
These late-onset genes do not significantly assist in predicting AD risk, but perhaps have more important roles in indicating the pathways involved and in identifying potential drug target [68]. Most of the confirmed risk genes act within the amyloid processing pathway. With the exception of APOE, most of the best candidate genes confer a very small relative risk of 1.2–1.5 [3]. Mutations in genes related to tau also result in dementias, but do not play a role in AD [72].
Diagnosis
Currently the most accurate way to diagnose AD is postmortem, through detection of pathology through immunohistochemical analysis of tissue samples. At best, clinical diagnosis can provide a probable diagnosis of AD. In addition to the standard neuropsychological testing, published diagnostic criteria are available for specifically detecting AD, such as those developed in 1984 by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ARDA) [57]. The NINCDS-ARDA criteria have over 80% sensitivity and specificity for distinguishing people with AD from those without dementia, although differentiating between AD and other dementias is less accurate (23–88% specificity) [10]. The accuracy can be increased through clinical judgment and medical examination to rule out other underlying conditions.

FIGURE 2-2 Promising diagnostic approaches currently in development.
A diagnosis of AD may also be strengthened through neuroimaging, of which the most commonly used and most accurate technique is Positron Emission Tomography (PET). PET is used to distinguish AD from other dementias, including early stage AD, through detection of reduced glucose metabolism in the brain [67]. Promising new neuroimaging techniques are currently undergoing testing in large clinical cohorts, including PET markers for AD pathology such as amyloid-β, and structural methods including volumetric calculation, measurement of cortical thickness, and three-dimensional brain mapping [70, 80, 89].
Both amyloid-β and tau are detectable as markers of AD in CSF and may be used in diagnosing AD. Analysis of CSF can be used to differentiate AD from other dementias, with a hallmark profile of reduced Aβ1-42 and higher levels of total tau or hyperphosphorylated tau [41, 54]. However, sampling of CSF is considered to be controversial in many countries due to its perceived risk and the discomfort associated with the procedure.
Diagnosis of AD therefore relies on a combination of different assessment methods, and a clear guidance on specific diagnostic criteria is needed. There is a large body of research currently ongoing to refine novel combinations of neuropsychological tests, neuroimaging, and biomarkers for AD, described in more detail in Fig. 2-2. In 2012 new diagnostic criteria for use in research were published with the aim of identifying people in different stages of AD, particularly for recruitment in clinical trials [28]. This is the first-time biomarkers have been included as part of operationalized clinical criteria for AD. The key items in the DuBois criteria are summarized in Fig. 2-3. Ongoing research will inform future decisions for guidance on diagnosis in clinical practice.
Treatment
Four pharmacological agents are available for the treatment of symptoms of AD. Three acetylcholinesterase inhibitors (AChEI), donepezil, rivastigmine, and galanthamine, are licensed for mild to moderate AD. These drugs enhance the levels of acetylcholine in the brain in order to protect cognitive pathways. Memantine, which is licensed for use in moderate to severe AD, acts to block NMDA receptors in the brain and prevents neuronal excitotoxicity, which is a hallmark of AD. While these medications provide some improvement in cognition, their efficacy is limited. Clinical trial data shows a modest benefit to cognition for 6–12 months with AChEI or memantine, and some improvement in mood and function of people with AD treated with AChEI [13, 51, 58, 93]. All of the current licensed treatments target the symptoms, rather than the underlying disease, in AD.

FIGURE 2-3 DuBois diagnostic criteria for Alzheimer’s disease (AD) in research. Probable AD is present where an individual fulfills the criteria in item A, plus one or more supportive features from criteria in items B, C, D, or E.
A number of novel disease-modifying drugs have been developed but are still in clinical trials. Most of these have focused on the amyloid cascade, including a number of immunotherapies designed to promote amyloid clearance. Despite promising indications in animal studies, early immunotherapies were disappointing, showing little impact on cognition and with some associated with severe side effects and encephalitis [38]. Following evidence of potential efficacy earlier in AD, modified immunotherapies with better safety profiles have now entered trials in people with early AD, and the findings are eagerly awaited. Other potential avenues for novel AD drugs include agents to selectively inhibit amyloid processing enzymes, prevent tau phosphorylation, for example, through glycogen synthase-kinase 3β inhibition, and target specific transcriptional factors involved in AD [85, 94]. However, most of these compounds are still in very preliminary testing.
A promising and complementary approach to drug development for AD is drug repositioning, a process involving the identification of existing treatments that could be repurposed for use in AD. A number of candidate drugs have been identified, including antibiotics, antihypertensives, and retinoids, which already have safety data and evidence to support a potential mechanism for treating AD [23
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree