Chapter 16 The Thalamus and Internal Capsule: Getting to and from the Cerebral Cortex
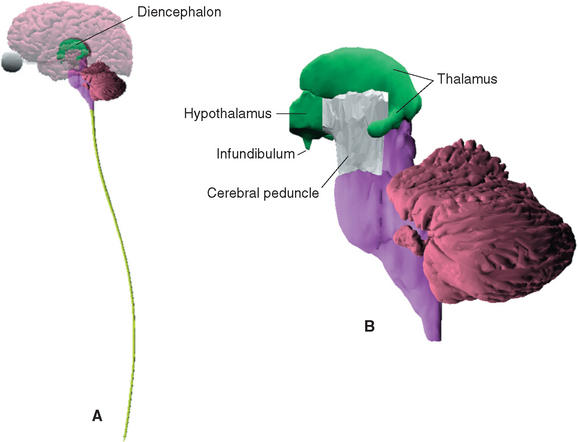
The diencephalon, mostly hidden from view between the cerebral hemispheres (Fig. 16-1A), constitutes only about 2% of the central nervous system (CNS) by weight. Nevertheless, it has extremely widespread and important connections, and the great majority of sensory, motor, and limbic pathways involve one or more stops in the diencephalon. Most motor and limbic pathways also involve telencephalic structures that are discussed in later chapters, so this chapter provides only a general overview of the connections of diencephalic nuclei. These connections are discussed in more detail in terms of functional systems in subsequent chapters, and a series of sections demonstrating major structures of both the diencephalon and the telencephalon is provided in Chapter 25. Nearly all the connections between the cerebral cortex and subcortical structures, prominently including the diencephalon, travel through the internal capsule, so an overview of this structure is provided here as well.
The Diencephalon Includes the Epithalamus, Subthalamus, Hypothalamus, and Thalamus
The diencephalon (Fig. 16-1B) is conventionally divided into four parts, each of which includes the term thalamus (from a Greek word meaning “inner chamber”) as part of its name.* These parts are (1) the epithalamus, which includes the pineal gland and a few nearby neural structures; (2) the dorsal thalamus, which is usually referred to simply as the thalamus; (3) the subthalamus; and (4) the hypothalamus.
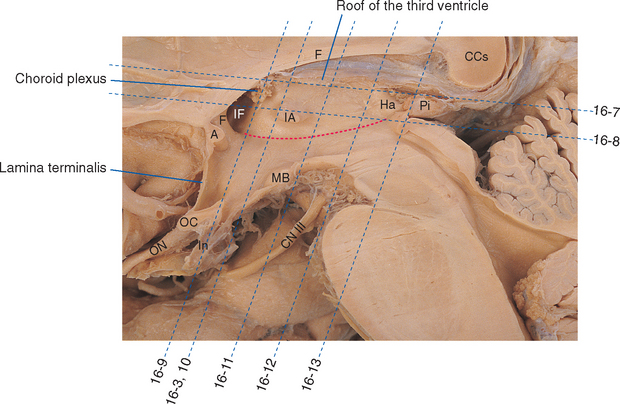
The only part of the diencephalon that can be seen on an intact brain is the inferior surface of the hypothalamus (see Figs. 3-16 and 3-17), which includes the mammillary bodies and the infundibular stalk. However, the entire medial surface of the diencephalon, much of which forms each wall of the third ventricle, can be seen on a hemisected brain (Fig. 16-2). Superiorly, the diencephalon borders the body of the lateral ventricle and the subarachnoid space of the transverse cerebral fissure; inferiorly, as previously noted, it is also exposed to subarachnoid space. Laterally, it is bounded by the internal capsule (Fig. 16-3). The caudal boundary of the diencephalon is a plane through the posterior commissure and the caudal edge of the mammillary bodies; the rostral boundary is a plane through the back of the anterior commissure and the front of the optic chiasm. These rostral and caudal boundaries are approximate and somewhat arbitrary and are used only for purposes of discussion. Functionally continuous neural tissue extends through both boundaries; in addition (as noted in earlier chapters), certain thalamic nuclei protrude through the posterior boundary to a position alongside the midbrain.
As a consequence of the cephalic flexure, the axis of the diencephalon is inclined about 80 degrees with respect to the axis of the brainstem (see Fig. 3-1). This means that sections cut in a plane similar to that used in the last few chapters (i.e., perpendicular to the long axis of the brainstem) are at a peculiar angle to the diencephalon. Therefore, in this and subsequent chapters, sections cut in axial and coronal planes are shown (Fig. 16-1).*
The Epithalamus Includes the Pineal Gland and the Habenular Nuclei
The pineal gland is a midline, unpaired structure situated just rostral to the superior colliculi. It resembles a pinecone in shape, which is how it got its name. Because each of us has only one pineal gland, which is located deep within the brain, it was once thought that this organ might be the seat of the soul. This now seems unlikely, because pineal tumors do not cause the changes one would expect to find with distortion of the soul; rather, these tumors compress the midbrain and cause the changes one would expect to see with distortion of this part of the brainstem. Early findings may include hydrocephalus (because the aqueduct gets squeezed shut) and various deficits in eye movements and pupillary reactions (because of damage to the oculomotor and trochlear nuclei and pathways ending in them). In addition, pineal tumors may cause changes in sexual development, giving a clue to at least one of its possible functions. The pineal arises as an evagination from the roof of the diencephalon; in fish, amphibians, and many reptiles, it contains photoreceptor cells similar to retinal cones. In these species it is suspected of monitoring day length and season and participating in the regulation of circadian and circannual rhythms (although there are probably other functions as well). The pineal gland of birds and mammals contains no photoreceptors and consists of a collection of secretory cells (pinealocytes), some glial cells, and a rich vascular network. Nevertheless, it still receives a light-regulated input by way of a circuitous pathway that begins in the retina and, after one or more relays in the hypothalamus, reaches the intermediolateral cell column of the spinal cord. Preganglionic sympathetic fibers from the spinal cord then synapse on postganglionic neurons of the superior cervical ganglion, which in turn send their axons to the pineal.
The mammalian pineal is an endocrine gland involved in seasonal cycles (e.g., reproductive cycles) and other functions and has no known neural output. Instead it secretes a hormone derived from serotonin, called melatonin, at relatively high rates during darkness. In many species melatonin has an antigonadotropic effect, and light, by way of the neural pathway just described, causes a decrease in melatonin production. As days get longer in the spring, melatonin production declines, which in turn causes an increase in gonadal function. This system is of considerable importance in mammals with prominent seasonal sexual cycles, but its effects in humans are not as clear. It has been reported, however, that nonparenchymal pineal tumors, which presumably destroy pinealocytes, tend to be associated with precocious puberty, as though the production of some antigonadotropic substance had been halted. The converse has been reported as well—that parenchymal pineal tumors tend to be associated with hypogonadism. These tumors are relatively rare, however, and in humans the pineal is probably more important in the regulation of circadian rhythms, including sleep-wake cycles (see Chapter 22). The routine clinical importance of the pineal arises from the fact that after the age of about 17 years, calcareous concretions accrue in it. This makes it opaque to x-rays and hence a useful radiological landmark (Fig. 16-4). Because it normally lies in the midline, slight shifts in pineal position can be indicative of expanding masses of various types.
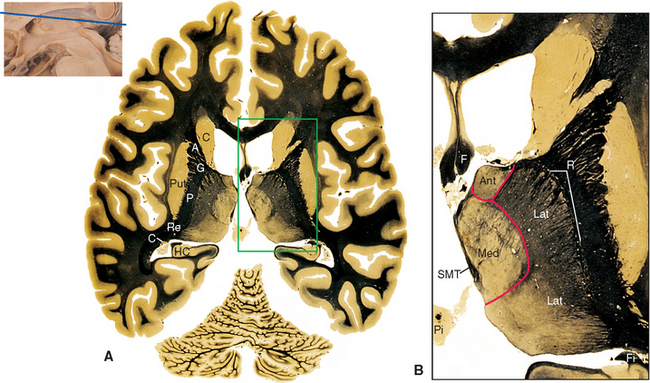
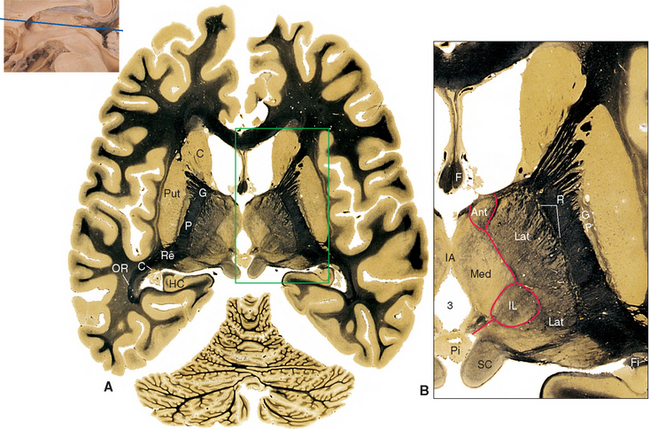
The pineal gland is attached to the dorsal surface of the diencephalon by a stalk. Caudally at the base of the stalk is the posterior commissure; rostrally is a small swelling on each side called a habenula (see Figs. 16-2 and 16-12). Underlying each habenula are the habenular nuclei. Each habenula receives one major input bundle, the stria medullaris (“white stripe”) of the thalamus, and gives rise to one major output bundle with the awesome name of habenulointerpeduncular tract (or fasciculus retroflexus). The stria medullaris of the thalamus (see Figs. 16-7 and 16-11) underlies a horizontal ridge on the dorsomedial surface of the thalamus to which the roof of the third ventricle is attached. The habenulointerpeduncular tract, as its name implies, extends from the habenula to the interpeduncular nucleus, located between the cerebral peduncles, and to other parts of the midbrain reticular formation. The fibers of the stria medullaris originate in the globus pallidus and some limbic structures, so the pathway through the habenula is one route through which the basal ganglia and limbic system can influence the brainstem reticular formation.
The Subthalamus Includes the Subthalamic Nucleus and the Zona Incerta
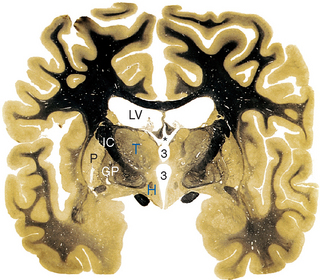
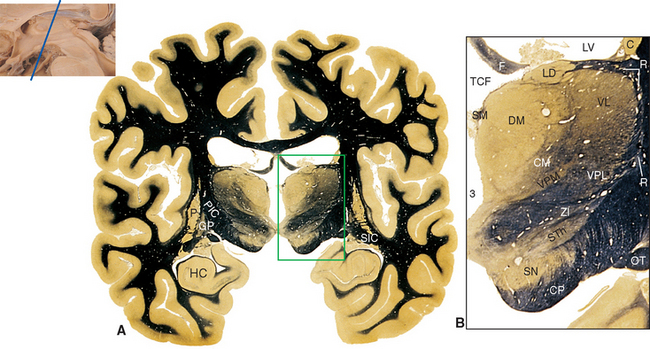
Parts of the midbrain tegmentum continue into the diencephalon as the subthalamus. This area is completely surrounded by neural tissue and is located inferior to the thalamus, lateral to the hypothalamus, and medial to the cerebral peduncle and internal capsule (see Figs. 16-11 and 16-12). The subthalamus contains rostral portions of the red nucleus and substantia nigra and is traversed by somatosensory pathways on their way to the thalamus, as well as by several pathways involving the cerebellum and basal ganglia (the latter pathways are discussed in Chapters 19 and 20). In addition, the subthalamus contains the subthalamic nucleus and zona incerta (see Fig. 16-11). The subthalamic nucleus is a lens-shaped, biconvex structure located just medial and superior to parts of the cerebral peduncle and internal capsule. This nucleus is interconnected with the basal ganglia, as discussed in Chapter 19. The zona incerta is a small mass of gray matter intervening between the subthalamic nucleus and the thalamus. It appears to be a rostral continuation of the midbrain reticular formation and has very widespread connections (including direct projections to the cerebral cortex), although its function is largely unknown.
The Thalamus Is the Gateway to the Cerebral Cortex
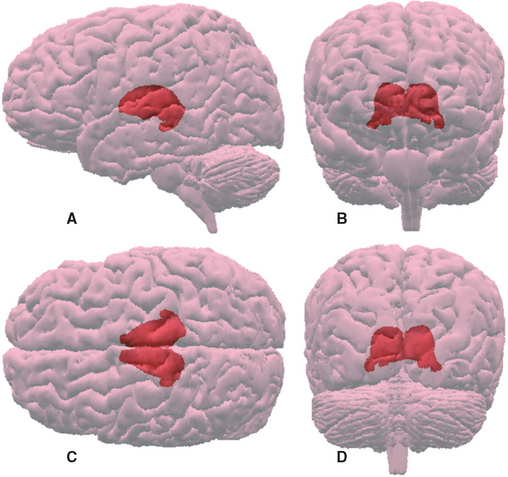
The thalamus is a large, egg-shaped, nuclear mass with a posterior appendage (Figs. 16-1B and 16-5), making up about 80% of the diencephalon. It extends anteriorly to the interventricular foramen, superiorly to the transverse cerebral fissure and the floor of the lateral ventricle, and inferiorly to the hypothalamic sulcus; posteriorly it overlaps the midbrain (see Fig. 16-13). The thalamus is part of a remarkably large number of pathways; all sensory pathways (other than olfaction) relay in the thalamus, and many of the anatomical circuits used by the cerebellum, basal ganglia, and limbic structures also involve thalamic relays. These various systems utilize more or less separate portions of the thalamus, which has therefore been subdivided into a series of nuclei.
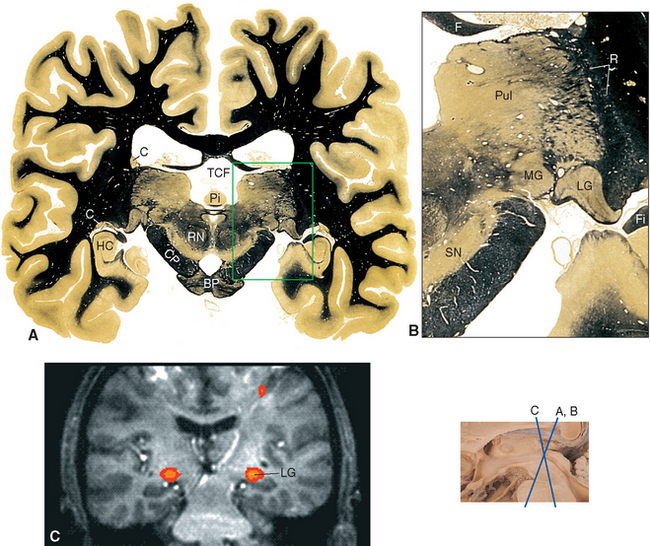
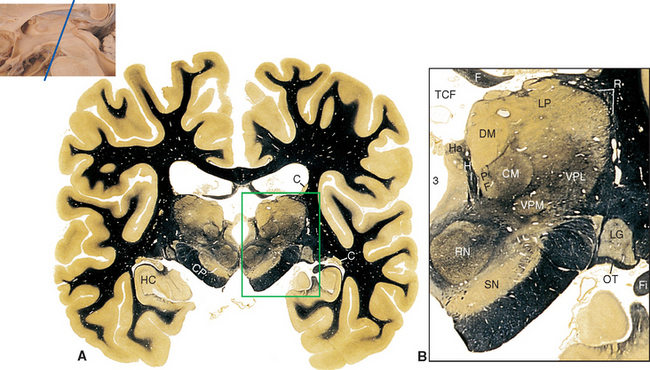
Figure 16-13 Coronal section through the posterior thalamus. A, The entire section shown at about 80% actual size. B, Enlargement of the area indicated in A, showing the lateral geniculate (LG), medial geniculate (MG), and reticular (R) nuclei and the pulvinar (Pul). C, Functional magnetic resonance imaging data from a subject watching a red and black checkerboard in which the squares reversed color 8 to 10 times per second, superimposed on a T1-weighted coronal slice at a level similar to that shown in A. The stimulus activates not only occipital cortex above and below the calcarine sulcus (see Fig. 6-21C), but also the lateral geniculate nucleus (LG). The inset at the bottom right shows the relative planes of section in A, B, and C. BP, basal pons; C, caudate nucleus; CP, cerebral peduncle; F, fornix; Fi, fimbria (fibers associated with the hippocampus that will join the fornix); HC, hippocampus; Pi, pineal gland; RN, red nucleus; SN, substantia nigra; TCF, transverse cerebral fissure.
(A and B, modified from Nolte J, Angevine JB Jr: The human brain in photographs and diagrams, ed 3, St. Louis, 2007, Mosby. C, from Chen W et al: Magn Reson Med 39:89, 1998.)
The Thalamus Has Anterior, Medial, and Lateral Divisions, Defined by the Internal Medullary Lamina
The topographical organization of the thalamus is shown in Figure 16-6 and Table 16-1. A thin, curved sheet of myelinated fibers, the internal medullary lamina, divides most of the thalamus into medial and lateral groups of nuclei (Figs. 16-7 and 16-8). Anteriorly, the internal medullary lamina splits and encloses an anterior group of nuclei, usually referred to collectively as the anterior nucleus, which borders on the interventricular foramen. The medial group similarly contains a single large nucleus, the dorsomedial (DM) nucleus (also commonly called the medial dorsal [MD] nucleus).
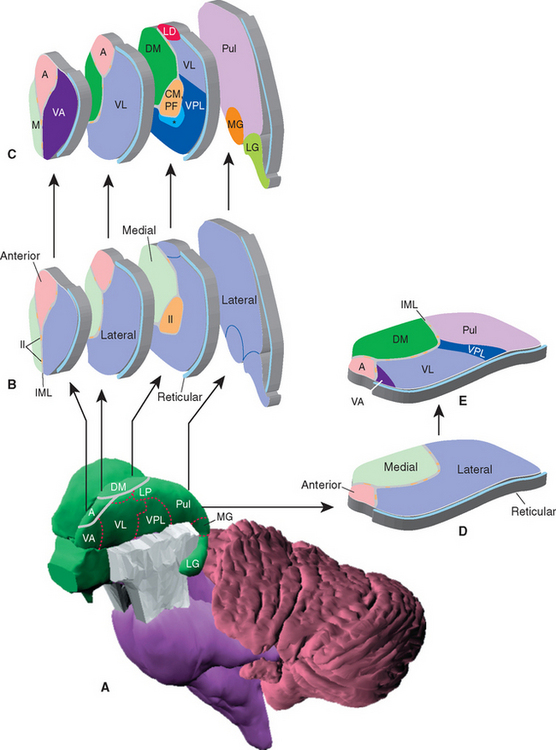
Figure 16-6 Topographical subdivisions of the thalamus. A, Lateral view of the left thalamus as seen from slightly above and in front. The reticular nucleus has been removed; ordinarily it would cover the entire lateral surface. B and C, Same view as A, but exploded into four pieces to show the internal arrangement of topographical subdivisions (B) and the major nuclei of each subdivision (C). The most anterior sliced surface corresponds approximately to Figure 16-9; the most posterior sliced surface corresponds approximately to Figure 16-13. D and E, A horizontal slab corresponding approximately to Figure 16-7 showing the internal arrangement of topographical subdivisions (D) and the major nuclei of each subdivision (E). *, ventral posteromedial nucleus; A, anterior nucleus; CM, centromedian nucleus (the largest intralaminar nucleus); DM, dorsomedial nucleus; Il, intralaminar nuclei; IML, internal medullary lamina; LD, lateral dorsal nucleus; LG, lateral geniculate nucleus; LP, lateral posterior nucleus; M, midline nuclei; MG, medial geniculate nucleus; PF, parafascicular nucleus; Pul, pulvinar; VA, ventral anterior nucleus; VL, ventral lateral nucleus; VPL, ventral posterolateral nucleus.
Table 16-1 Topographical Subdivisions of the Thalamus and Their Principal Nuclei
| Subdivision | Principal Nuclei | Common Abbreviation |
|---|---|---|
| Anterior division | Anterior | |
| Medial division | Dorsomedial (medial dorsal) | DM (MD) |
| Lateral division | Dorsal tier | |
| Lateral dorsal | LD | |
| Lateral posterior | LP | |
| Pulvinar | ||
| Ventral tier | ||
| Ventral anterior | VA | |
| Ventral lateral | VL | |
| Ventral posterior | VP | |
| Ventral posterolateral | VPL | |
| Ventral posteromedial | VPM | |
| Medial geniculate | MGN | |
| Lateral geniculate | LGN | |
| Intralaminar nuclei | Centromedian | CM |
| Parafascicular | PF | |
| Others | ||
| Reticular nucleus | Reticular nucleus |
The lateral group of nuclei composes the bulk of the thalamus and is further subdivided into a dorsal tier and a ventral tier. The dorsal tier consists of the lateral dorsal (LD) nucleus (see Fig. 16-11), the lateral posterior (LP) nucleus (see Fig. 16-12), and the large pulvinar (see Fig. 16-13). The lateral posterior nucleus is continuous with the pulvinar; both nuclei have similar connections, so the two together are sometimes referred to as the pulvinar-LP complex. The bulk of the ventral tier consists of three nuclei arranged in an anterior-posterior sequence: the ventral anterior (VA) nucleus (Fig. 16-9), the ventral lateral (VL) nucleus (Figs. 16-10 and 16-11), and the ventral posterior (VP) nucleus (Figs. 16-11 and 16-12). The ventral posterior nucleus is customarily subdivided into the ventral posterolateral (VPL) nucleus and the ventral posteromedial (VPM) nucleus. VPL is the somatosensory relay nucleus for the body, and VPM serves the same function for the head. VA and VL are involved in motor control circuits that include the cerebellum and basal ganglia. The lateral geniculate nucleus (visual system) and medial geniculate nucleus (auditory system) are located posterior to these ventral tier nuclei and inferior to the pulvinar, and protrude posteriorly alongside the midbrain (Fig. 16-13).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree