FIGURE 15.1 Section through the pons at the level of the trigeminal nuclei.
The Motor Portion
Upper motor neuron control of trigeminal motor functions arises primarily from the lower third of the contralateral motor cortex, although each trigeminal motor nucleus receives projections from both cerebral hemispheres. Fibers descend in the corticobulbar tract to the pons, where they decussate (Figure 15.2). There is extrapyramidal innervation from the premotor cortex and basal ganglia. The muscles supplied by the trigeminal are derived from the first branchial arch, and the system is special visceral efferent (SVE) or branchial motor. The fibers exit laterally, typical for SVE fibers, but do not form an internal loop as other branchial motor fibers do.

FIGURE 15.2 The trigeminal nerve and its connections.
The motor root exits the lateral pons anteromedial to the sensory root. It passes beneath the gasserian ganglion, leaves the skull through the foramen ovale, and then joins the mandibular sensory division briefly before separating to supply the muscles of mastication and associated muscles. Techniques for trigeminal motor nerve conduction studies have been described.
The principal function of the motor root is to innervate the muscles of mastication: masseter, temporalis, and medial and lateral pterygoids. The masseter muscles close the jaw and protrude it slightly; the masseter may be the most powerful muscle in the body. The temporalis muscles close the jaw and retract it slightly. The medial pterygoids acting synchronously close the jaw and protrude it. The lateral pterygoids acting synchronously open the jaw and protrude it. The medial and lateral pterygoids originate from the skull base and extend laterally to insert on the inner aspect of the mandible. When they contract on one side, they pull the mandible contralaterally. When there is unilateral pterygoid weakness, the jaw deviates toward the side of the weak muscles.
Mastication is a complex opening, closing, forward, backward, and lateral movement of the jaw. The motor root of CN V is responsible for all of these intricate motions. Disturbed central programming of mastication with “inverse masticatory muscle activity” has been reported in syringobulbia. CN V also supplies the mylohyoid, anterior belly of the digastric, tensor veli palatini, and tensor tympani muscles. The mylohyoid pulls the hyoid bone upward and forward, raising the floor of the mouth and pressing the base of the tongue against the palate. The anterior belly of the digastric raises and advances the hyoid bone if the jaw is fixed. The tensor veli palatini tenses the soft palate and helps prevent food from escaping from the oro-to the nasopharynx; it also dilates the eustachian tube. The tensor tympani, through interaction with CN VIII, tenses the tympanic membrane and helps dampen its excursions in response to sound intensity.
The Sensory Portion
The trigeminal, or gasserian (for J. L. Gasser), ganglion, the largest ganglion in the peripheral nervous system, lies just beside the pons in a shallow depression in the petrous apex called Meckel’s cave. The ganglion is crescent shaped, convex anterolaterally, and is also known as the semilunar ganglion. It lies just lateral to the internal carotid artery and the posterior part of the cavernous sinus. The ganglion is analogous to a dorsal root ganglion; it contains unipolar sensory neurons, whose central processes enter the lateral pons through the large sensory root that passes beneath the tentorium to connect the concave side of the ganglion to the brainstem. The sensory root can be compressed by vascular loops, causing trigeminal neuralgia (TN). The peripheral processes subserve sensation to the face and head. There are two types of sensory neurons in the gasserian ganglion. One mediates fine discriminative touch; the other mediates primarily pain and temperature.
Afferent fibers conveying light touch and pressure enter the principal sensory nucleus, which lies in the tegmentum just lateral and posterior to the motor nucleus; most fibers synapse there and give rise to second order neurons that cross the midline and ascend in the ventral trigeminothalamic tract en route to the ventral posterior medial (VPM) thalamic nucleus (Figure 15.2). Some fibers ascend ipsilaterally in the small dorsal trigeminothalamic tract to VPM. The two sets of trigeminothalamic fibers, both of which run near the medial lemniscus, are sometimes referred to as the trigeminal lemniscus.
Fibers subserving pain and temperature take a much more circuitous route to the thalamus. The spinal tract, or the descending root, of the trigeminal extends from the principal sensory nucleus down through the lower pons and medulla, into the spinal cord as far as C3, or even C4 (Figure 15.2). There the spinal tract becomes continuous with Lissauer’s tract. The nucleus of the spinal tract is a cell column that lies just medial to the fiber tract throughout its course. In the cervical cord, the nucleus of the spinal tract becomes continuous with the substantia gelatinosa of the posterior horn. Fibers conveying pain and temperature enter the spinal tract of the trigeminal, and descend to various levels depending on their somatotopic origin, then synapse in the adjacent nucleus of the spinal tract. The axons of second order neurons cross the midline, aggregate in the ventral trigeminothalamic tract, and ascend to VPM alongside the medial lemniscus and spinothalamic tracts. Fibers arising from the pars caudalis send collaterals to the intralaminar and posterior thalamic nuclei. From VPM, fibers project through the thalamic radiations to the sensory cortex in the postcentral gyrus, where facial sensation occupies the lower third. Some projections from VPM terminate in the precentral gyrus. Fibers from the intralaminar nuclei project well outside the primary sensory cortex. Sensory fibers from CNs VII, IX, and X provide sensation to the region of the external ear canal; these fibers join the trigeminal system centrally.
The somatotopic organization of the nucleus and spinal tract is complex. There are three subnuclei, from above to below—the nuclei (or pars) oralis, interpolaris, and caudalis. The pars oralis extends from midpons to the level of the inferior olive, the pars interpolaris from the inferior olive to the obex, and the pars caudalis from there to the upper cervical cord. At one time it was thought that different divisions of the trigeminal descended to different levels in the spinal tract. This concept was based in part on the alterations in corneal sensation after surgical tractomy of the cervical cord, which is done to treat chronic pain. Current thinking is that all three divisions are represented at all levels of the nucleus, although V1 may not project as far caudally as V2 and V3. Somatotopically, V1 is represented most anteriorly, and V2 and V3 more posteriorly (resembling a small inverted face with the forehead anterior on a typical cross section).
Dejerine, using clinical and pathological material, demonstrated an “onion skin” somatotopic organization (Figure 15.3). The face is represented as concentric rings from the perioral region to the preauricular region. Fibers from the foreface (upper lip, mouth, and tip of the nose) synapse most rostrally in the nucleus of the spinal tract; those from the hindface synapse more caudally, adjacent to the sensory input from C2 and C3. Because of this organization there is occasionally sparing, less frequently selective involvement of the perioral region compared to the posterior face (balaclava helmet distribution). The onionskin distribution is important in understanding the patterns of facial sensory loss that may occur with intrinsic brainstem and cervical spinal cord lesions, especially syringomyelia and syringobulbia. Chang recently reported a clinical demonstration of the onionskin organization in a case of central cord syndrome where facial dysesthesias retreated from the center toward the periphery. Onionskin sensory loss was apparently common in neurosyphilis.
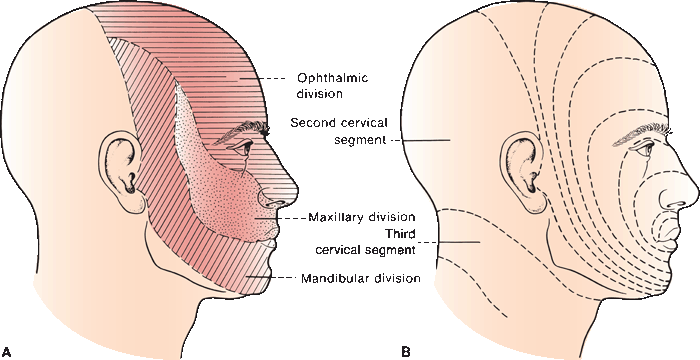
FIGURE 15.3 Cutaneous distribution of the trigeminal nerve. A. Peripheral distribution. B. Segmental distribution.
The third sensory component, the mesencephalic root of the trigeminal nerve, runs with the motor root and then extends posteriorly and cephalad from the level of the motor nucleus into the mesencephalon. It carries proprioceptive impulses from the muscles supplied by the trigeminal nerve and probably for the extraocular muscles and the muscles of facial expression as well. Neurons subserving proprioception are unipolar neurons, but they reside inside the brainstem in the mesencephalic nucleus of CN V, making it in essence an ectopic dorsal root ganglion within the central nervous system (CNS). Proprioceptive fibers pass through the gasserian ganglion without synapsing and terminate in the mesencephalic nucleus. The mesencephalic nucleus mediates the jaw jerk reflex. Projections join the trigeminothalamic tracts and ascend to VPM.
The Trigeminal Divisions
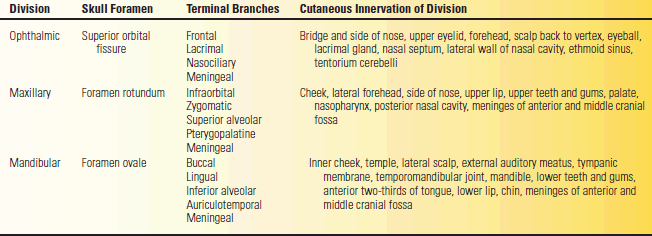
The three divisions of CN V arise from the trigeminal ganglion. Each division has a meningeal branch. Disregarding these, the ophthalmic division has three major terminal branches; the other two divisions have four each. The terminal branches of V1 are the frontal, lacrimal, and nasociliary nerves. The terminal branches of the maxillary division are the infraorbital, zygomatic, superior alveolar, and pterygopalatine. The terminal branches of the mandibular division are the buccal, lingual, inferior alveolar, and auriculotemporal. The cutaneous distribution of the divisions is summarized in Table 15.1.
TABLE 15.1 The Divisions of the Trigeminal Nerve, the Foramina Through Which They Pass, Their Terminal Branches, and Fields of Cutaneous Innervation
From the gasserian ganglion, V1—the smallest of the three divisions—runs forward and enters the cavernous sinus; it lies laterally in the wall of the sinus between the folds of dura (Figure 14.6). A branch is given off to the meninges of the tentorium cerebelli just after leaving the ganglion. CN V1 runs forward through the superior orbital fissure and divides into its terminal branches. The sensory innervation of V1 is shown in Figure 15.3. Note that V1 supplies most of the nose. Sensory loss along the nose due to a lesion of the distal branches of V2 is shown in Figure 15.5. The sensory fibers to the eye pass through the ciliary ganglion without synapsing and continue as the short ciliary nerves; these convey sensation from the globe and carry postganglionic sympathetic fibers to the pupilloconstrictor muscle. The long ciliary nerves carry sensation from the ciliary body and cornea, as well as sympathetic fibers to the pupillodilator muscle. Proprioceptive fibers from the extraocular muscles travel initially with their respective CNs, but join V1 and proceed to the mesencephalic nucleus.
The maxillary branch gives off the middle, or recurrent, meningeal nerve to the dura of the middle fossa, passes through the lateral wall of the cavernous sinus, and then exits through the foramen rotundum. It crosses the pterygopalatine (sphenopalatine) fossa, where the sensory branches to the palate are given off, after which the nerve splits into the zygomatic and posterior superior alveolar branches. The palatine nerves traverse the sphenopalatine ganglion without synapsing to innervate the hard and soft palate. The nerve enters the orbit via the inferior orbital fissure and transits the infraorbital canal. The middle and anterior alveolar branches arise in the infraorbital canal. The anterior alveolar branch exits through the infraorbital foramen and becomes the infraorbital nerve. Lesions of the infraorbital nerve cause the numb cheek syndrome (see below).
The mandibular division, the largest of the branches, gives off a small meningeal branch, and then exits through the foramen ovale. It runs for a short distance with the motor root, forming a large trunk. This trunk gives off the nervous spinosus and the branch to the medial pterygoid muscle. The nervous spinosus is a recurrent twig that re-enters the skull through the foramen spinosum and runs alongside the middle meningeal artery to innervate the meninges of the anterior and middle fossa. The trunk then divides into a small, anterior, chiefly motor branch and a large, posterior, chiefly sensory branch. The sensory filaments of the anterior branch form the buccal nerve. The posterior branch divides into three large terminal nerves. Two, the lingual and auriculotemporal, are purely sensory. The third, the inferior alveolar, also carries motor fibers to the mylohyoid and anterior belly of the digastric. The lingual nerve carries somatic sensation from the anterior two-thirds of the tongue. Taste sensation from the same region is carried by the chorda tympani and CN VII. After the origin of the lingual nerve, the nerve enters the mandibular foramen, traverses the mandibular canal and emerges through the mental foramen as the mental nerve to supply sensation to the chin. Lesions of the mental nerve produce the numb chin syndrome (see below).
Some specifics about the trigeminal sensory innervation are clinically noteworthy. The innervation of the cornea is generally said to be CN V1, although there is some evidence that the upper cornea may be CN V1 and the lower cornea CN V2, at least in some patients. CN V1 innervates most of the nose, including the nasal septum. CN V1 territory extends back to the scalp vertex; it does not stop at the hairline. Figure 15.4 demonstrates the territory of the ophthalmic division outlined by postzoster scarring. CN V2 innervates the inferior lateral aspect of the nose and the cheek. The cutaneous distribution of CN V2 is nearly identical to the infraorbital nerve (Figure 15.5). Changes in sensation involving the upper teeth and gums can be helpful in distinguishing a CN V2 lesion from an infraorbital nerve lesion in patients with the numb cheek syndrome. The major terminal branch of CN V3 is the mental nerve, which provides sensation to the chin and lower lip. The distribution of CN V3 does not extend to the jaw line; there is a large “notch” at the angle of the jaw innervated by the greater auricular nerve (C2-3). This notch of C2-3 innervation can be surprisingly large (Figure 15.6).
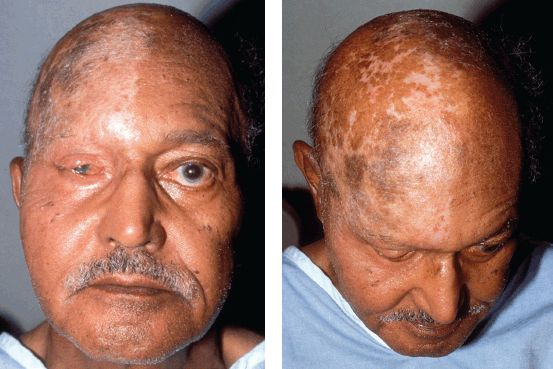
FIGURE 15.4 A remote case of ophthalmic division zoster has left severe postinflammatory scarring, which outlines the cranial nerve (CN) V1 distribution. Note the scarring extends back to the interaural line and involves much of the nose.
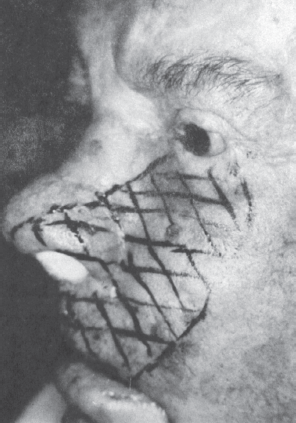
FIGURE 15.5 Patient with infraorbital neuropathy from carcinomatous infiltration. Note that the maxillary division innervates only the side of the nose distally. This patient had numbness of only the anterior teeth and gums, which proved the lesion was at the infraorbital foramen and not intracranial. (From Campbell WW. The numb cheek syndrome: a sign of infraorbital neuropathy. Neurology 1986;36:421–423.)
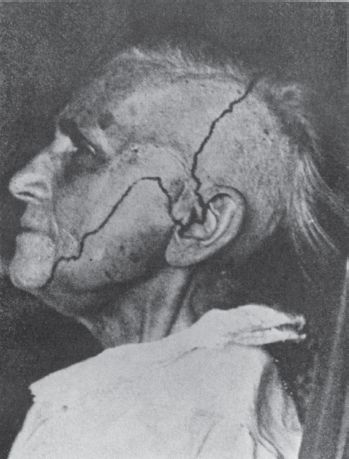
FIGURE 15.6 The distribution of sensory loss following complete section of the trigeminal root. Note the large area at the angle of the jaw that is innervated by C2 through the greater auricular nerve, and the inclusion of the tragus of the ear in the trigeminal distribution.
CN V supplies filaments to four ganglia in the head: the ciliary, sphenopalatine, otic, and submaxillary (Box 15.1).
The Ciliary, Sphenopalatine, Otic, and Submaxillary Ganglia
The ciliary ganglion, located in the posterior orbit, receives sensory fibers from the nasociliary branch of cranial nerve (CN) V1 (the long root of the ciliary ganglion), parasympathetic fibers from the Edinger-Westphal nucleus through the inferior division of CN III (the short root), and sympathetic fibers from the cavernous sympathetic plexus, running through the long ciliary nerves. Its branches, the short ciliary nerves, supply the ciliary muscle, sphincter and dilator of the pupil, and cornea.
The sphenopalatine ganglion, located in the pterygopalatine fossa, receives sensory fibers from the sphenopalatine branches of CN V2, parasympathetic fibers from the nervus intermedius via the greater superficial petrosal nerve, and sympathetic fibers from the pericarotid plexus through the deep petrosal nerve. The deep and greater superficial petrosal nerves join to form the vidian nerve, or nerve of the pterygoid canal, before entering the ganglion. The sphenopalatine ganglion sends branches to the posterior ethmoidal and sphenoidal sinuses, the hard and soft palates, tonsils, uvula, nasal mucosa, and nasopharynx. Lacrimal fibers pass along the zygomaticotemporal branch of CN V2 to the lacrimal branch of CN V1, then to the lacrimal gland.
The otic ganglion, located in the infratemporal fossa just below the foramen ovale, receives a motor and possibly a sensory branch from CN V3, parasympathetic and sensory fibers from CN IX through the lesser superficial petrosal nerve, and sympathetic fibers from the plexus surrounding the middle meningeal artery. It sends motor branches to the tensor tympani and tensor veli palatini muscles and secretory fibers to the parotid gland through the auriculotemporal nerve.
The submaxillary ganglion, located near the submaxillary gland, receives sensory fibers from the lingual branch of CN V3, parasympathetic fibers from the superior salivatory nucleus of CN VII through the chorda tympani, and sympathetic fibers from a plexus around the external maxillary artery. It sends secretory fibers to the submaxillary and sublingual glands and the mucous membrane of the mouth and tongue.
CLINICAL EXAMINATION
Examination of the Motor Functions
Assessment of trigeminal motor function is accomplished by examining the muscles of mastication. Bulk and power of the masseters and pterygoids can be gauged by palpating these muscles as the patient clinches the jaw. An effective technique is to place the examining fingers along the anterior, not lateral, border of the masseters bilaterally. When the jaw is clenched, the fingers will move forward; this movement should be symmetric on the two sides. Unilateral trigeminal motor weakness causes deviation of the jaw toward the weak side on opening, due to the unopposed action of the contralateral lateral pterygoid. The tongue also deviates toward the side of the weakness with CN XII lesions. So both the tongue and the jaw deviate toward the weakness. Whether this is toward or away from the lesion depends on the specifics of the lesion. Figure 15.7 shows a patient with both tongue and jaw deviation. For a video of a patient with jaw deviation, see http://www.youtube.com/watch?v=bTAQa-ZKMuQ&feature=autoplay&list=ULvY3OtzzW6C4&index=127&playnext=. Careful observation of jaw opening is often the earliest clue to the presence of an abnormality. It is occasionally difficult to be certain whether the jaw is deviating or not. Note the relationship of the midline notch between the upper and lower incisor teeth; it is a more reliable indicator than lip movement. The tip of the nose and the interincisural notches should line up. A straightedge against the lips can help detect deviation. Another useful technique is to draw a vertical line across the midline upper and lower lips using a felt-tip marker. Failure of the two vertical marks to match when the jaw is opened indicates deviation. If there is any suggestion of a problem, have the patient move the jaw from side to side. With unilateral weakness, the patient is unable to move the jaw contralaterally. To review, weakness of the right pterygoids causes deviation of the jaw to the right on spontaneous opening, and inability to move the jaw to the left on command. With facial weakness there may be apparent deviation of the jaw, and of the tongue, because of the facial asymmetry. Holding up the weak side manually will sometimes eliminate the pseudodeviation.
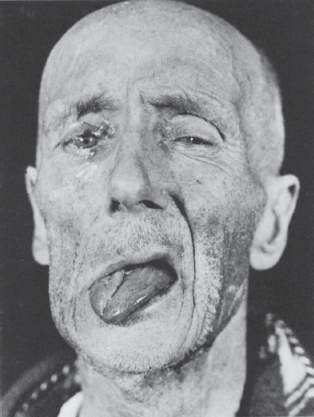
FIGURE 15.7 Infranuclear paralysis of the right trigeminal, facial, and hypoglossal nerves in a patient with metastatic carcinoma, showing deviation of the tongue and mandible to the right.
Other techniques for examining trigeminal motor function include having the patient protrude and retract the jaw, noting any tendency toward deviation, and having the patient bite on tongue depressors with the molar teeth, comparing the impressions on the two sides and comparing the difficulty of extracting a tongue depressor held by the molar teeth on each side.
Unilateral weakness of CN V–innervated muscles generally signifies a lesion involving the brainstem, gasserian ganglion, or the motor root of CN V at the base of the skull. Severe bilateral weakness of the muscles of mastication with inability to close the mouth (dangling jaw) suggests motor neuron disease, a neuromuscular transmission disorder, or a myopathy. With significant atrophy of one masseter, a flattening of the jowl on the involved side may be apparent (Figure 15.8). With temporalis atrophy there may be a hollowing of the temple. Rarely, fasciculations or other abnormal involuntary movements occur. There is no reliable or realistic method for examination of the other muscles supplied by CN V. Paralysis of the tensor tympani may cause difficulty hearing high notes. Because of bilateral innervation, unilateral upper motor neuron lesions rarely cause significant impairment of trigeminal motor function. There may be mild, transitory unilateral weakness. The amount of involvement depends on the extent of decussation. In bilateral supranuclear lesions, there may be marked paresis.
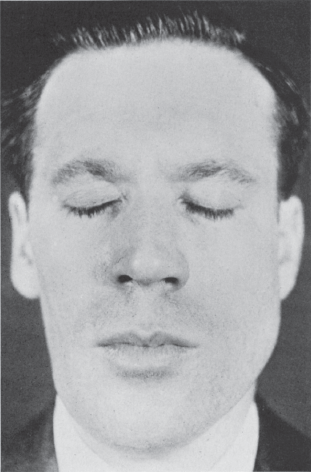
FIGURE 15.8 Infranuclear paralysis of the right trigeminal nerve with atrophy of the muscles of mastication.
Examination of the Sensory Functions
In testing facial sensation, touch, pain, and occasionally temperature are examined in the same manner as elsewhere on the body (Chapter 32), searching for areas of altered sensation. It is better to ask the patient if the stimuli feel the same on the two sides, rather than suggesting they might feel different. Sensation should be compared in each trigeminal division, and the perioral region compared to the posterior face to exclude an onionskin pattern. Pain or temperature should be compared with touch to exclude dissociated sensory loss (a common finding in lateral medullary syndrome). Sometimes it is useful to examine the nostrils, gums, tongue, and insides of the cheeks. Proprioception cannot be adequately tested, but one can test for extinction and the ability to identify figures written on the skin.
There are three common exercises in evaluating facial sensation: (a) determining whether sensory loss is organic or nonorganic, (b) determining which modalities are involved, and (c) defining the distribution. Complaints of facial numbness are common, and not all are organic. However, real facial sensory loss can be a serious finding, occasionally signifying underlying malignancy. The various methods and tricks for detecting nonorganic sensory loss are not entirely reliable, and this diagnosis should be made with caution. Patients with nonorganic sensory loss may have a demarcation of the abnormal area at the hairline rather than the scalp vertex. On the lower face, functional sensory loss tends to follow the jaw line and involve the notch over the masseter muscle, which is not trigeminal innervated (Figure 15.6). However, patients with intramedullary lesions may have involvement of the angle of the jaw. On the trunk, organic sensory loss typically stops short of midline because of the overlap from the opposite side, and splitting of the midline suggests nonorganicity. This finding is not reliable on the face because there is less midline overlap, so organic facial sensory loss may extend to the midline. The corneal and sternutatory reflexes (see below) should be normal in nonorganic sensory loss. Splitting of vibration along the midline is reputedly a nonorganic sign. Because the frontal bone and mandible are single bones, there should be no difference in vibratory sensibility on either side of midline. Patients who report a difference in vibratory sensibility on testing just to either side of midline may have nonorganic sensory loss. The reliability of this sign has not been validated; it can be misleading. Other signs suggestive of nonorganicity include dissociation between pinprick and temperature, variability from trial to trial, history of hypochondriasis, secondary gain, la belle indifference, nonanatomical sensory loss, and changing boundaries of hypalgesia. Gould et al. have appropriately cautioned about the validity of hysterical signs and symptoms.
Examination of the Reflexes
The corneal, sternutatory, and jaw reflexes are the reflexes most often assessed in evaluating the trigeminal nerve. Many other reflexes have been described, but they are of limited value and are seldom used. Some are archaic. These other reflexes are described in Table 15.2. The afferent limbs of these reflexes are trigeminal mediated. In some, the efferent limb is also trigeminal (e.g., the jaw jerk); in others, the efferent limb is executed through connections with CN III, CN VII, or other pathways.
TABLE 15.2 Trigeminal Mediated Reflexes
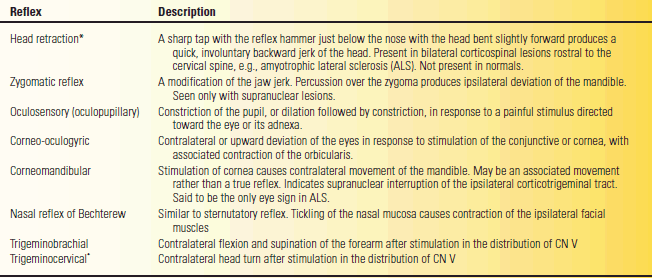
*Short-latency trigeminocervical responses can be recorded from sternocleidomastoid muscle after stimulation of the trigeminal nerve as an electrophysiologic counterpart of the head retraction reflex; these are not the same as the trigeminocervical reflex elicited by physical examination.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







