Tiagabine
Steven C. Schachter
HISTORICAL BACKGROUND AND CHEMISTRY
Tiagabine (-)-(R)-1-[4,4-Bis(3-methyl-2-thienyl)-3-butenyl] nipecotic acid hydrochloride (TGB; Gabitril, Abbot Laboratories, Cephalon, Inc. West Chester, Pennsylvania) received regulatory clearance from the US Food and Drug Administration (FDA) for the adjunctive treatment of partial seizures in adults and children 12 years and older in October 1997. TGB was synthesized by using an aliphatic chain to link nipecotic acid to a lipophilic anchor (Fig. 64.1). Nipecotic acid is effective against seizures in animal models only when injected into the cerebral ventricles, because it does not cross the blood-brain barrier (BBB) (1). The lipophilic anchor allows the attached nipecotic acid to readily cross the BBB.
MECHANISM OF ACTION
TGB blocks the neuronal and glial reuptake of gamma-aminobutyric acid (GABA) after its release from postsynaptic GABA receptors, thereby enhancing GABA-mediated inhibition at central nervous system (CNS) sites (2,3). Accordingly, TGB suppresses hyperexcitability in the dentate gyrus and CA3 area in epileptic E1 mice (4).
TGB binds to the GABA uptake carrier GAT-1 in animals (5,6) and postmortem human brain tissue (7,8), but not to any other neurotransmitter uptake sites or receptors. TGB has no significant effect on sodium or calcium channels (5,9).
Animal Seizure Models
TGB reduces the severity and duration of convulsions in amygdala-kindled rats (5) and significantly retards kindling (10). In addition, TGB reduces maximal electroshock-induced seizures and bicuculline-induced seizures in rats (5), and picrotoxin-induced convulsions in mice (11). The agent partially protects against photically induced myoclonus in photosensitive baboons (5) and blocks audiogenic convulsions in genetically epilepsy-prone rats in a dose-dependent manner (12).
PHARMACOKINETICS
Absorption and Distribution
TGB is rapidly and almost completely absorbed (17). Oral bioavailability is approximately 90%, and the absorption of TGB is linear over the therapeutic dosage range. Maximum serum concentrations are attained within 45 to 90 minutes in the fasting state and after a mean of 2.6 hours when taken with food (9). The extent of TGB absorption is not affected by food.
Metabolism and Elimination
Extensive oxidation of TGB occurs in the liver via isoform 3A of the cytochrome P450 (CYP450) family of enzymes (18). Only 2% of the administered dose is excreted as parent drug. The E- and Z-5-oxo-tiagabine isomers are the prominent metabolites in plasma and urine. Two major metabolites in human feces remain unidentified. TGB elimination is linear over the therapeutic dosage range (19).
The half-life of TGB is 5 to 8 hours in patients with uninduced liver function (20) and 2 to 3 hours in patients taking hepatic enzyme-inducing antiepileptic drugs (AEDs) (9,19). More frequent dosing does not appear to be necessary to compensate for the shortened half-life, however. TGB 32 mg per day, as add-on therapy, is equally effective whether administered as a 16-mg dose twice daily or as an 8-mg dose four times per day (21).
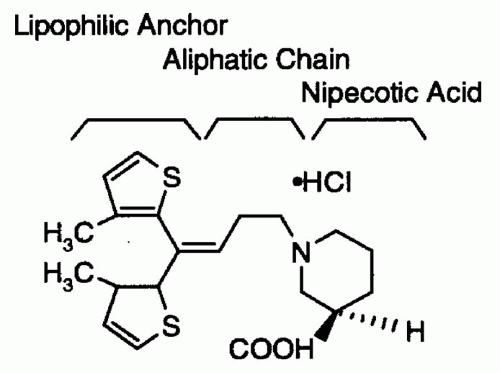 Figure 64.1 Chemical structure of tiagabine. (From Schachter SC. Tiagabine: current status and potential clinical applications. Exp Opin Invest Drugs 1996;5:1377-1387.) |
When adjusted for body weight, TGB elimination is two times higher in children than in uninduced adults with epilepsy (22). The pharmacokinetics of TGB are similar in healthy elderly volunteers and healthy young volunteers (23).
Drug Interactions
Concurrently administered drugs that enhance the activity of CYP3A increase the clearance of TGB and decrease the half-life of the agent. Therefore, TGB serum concentrations may increase if treatment with concomitantly administered enzyme-inducing AEDs is discontinued (26).
Because TGB neither induces nor inhibits the hepatic microsomal enzymes involved in drug metabolism, it minimally affects the serum concentrations of other agents (20). Moreover, although TGB is 96% protein bound, its serum concentration is too low to cause significant displacement of other protein-bound AEDs from albumin.
The metabolism of oral contraceptives is unaffected by TGB 8 mg per day (27), but whether higher doses of the agent have an effect on oral contraceptive metabolism is unknown.
EFFICACY
Add-on Studies of Patients with Refractory Partial Seizures
Five multicenter, double-blind, randomized, placebo-controlled studies evaluated TGB for the adjunctive treatment of partial-onset seizures in 951 patients, 675 of whom were randomly assigned to receive TGB (28, 29, 30, 31). Three pivotal studies with parallel-group, add-on designs (21,32,33) enrolled patients taking one to three concomitant hepatic enzyme-inducing AEDs. The dose-response study compared the efficacy of three different doses of TGB (16, 32, and 56 mg per day) with that of placebo (32). The thrice-daily dosing study compared the efficacy of TGB 10 mg administered three times per day with that of placebo (33).
In the dose-response study, the reduction in median seizure rates was statistically significant for both higher dosage groups (32 mg and 56 mg) compared with the placebo group (32). In the thrice-daily dosing study, TGB 10 mg administered three times per day was significantly more effective than placebo in reducing 4-week complex partial seizure (CPS) rates and simple partial seizure rates from baseline (33).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






