Tourette syndrome (TS) is a neurologic disorder manifested by motor, vocal, or phonic tics that, in most cases, start in childhood and are often accompanied by obsessive–compulsive disorder (OCD), attention-deficit/hyperactivity disorder (ADHD), poor impulse control, and other comorbid behavioral problems (1,2). Once considered a rare psychiatric curiosity, TS is now recognized as a relatively common and complex neurobehavioral disorder. Many notable historical figures, including Dr. Samuel Johnson and possibly Wolfgang Amadeus Mozart, are thought to have been afflicted with TS (3).
The clinical expression of this genetic disorder varies from one individual to another, fluctuations in symptoms are seen within the same individual, and different manifestations occur in various family members. This variable expression from one individual to another, even within members of the same family, contributes to diagnostic confusion. Without a specific biologic marker, the diagnosis depends on a careful evaluation of the patient’s symptoms and signs by an experienced clinician. However, many patients remain undiagnosed, or their symptoms are wrongly attributed to habit, allergies, asthma, dermatitis, hyperactivity, nervousness, and many other conditions (4,5).
PHENOMENOLOGY OF TICS
Tics, the clinical hallmark of TS, are relatively brief and intermittent movements (motor tics) or sounds (vocal or phonic tics). The term “phonic tic” is preferable because not all sounds produced by TS patients involve the vocal cords. Although both types of tics must be present for the diagnosis of TS, this division into motor and phonic tics is arbitrary because phonic tics are actually motor tics involving respiratory, laryngeal, pharyngeal, oral, and nasal musculature. Motor tics typically consist of sudden, often repetitive, movements, gestures, and utterances that mimic fragments of normal behavior. To understand better the categorization of tics and how they fit in the general schema of movement disorders, it may be helpful to provide a simple classification of movements. All movements can be categorized into one of four classes:
1. Voluntary: (a) intentional (planned, self-initiated, internally generated) and (b) externally triggered (in response to some external stimulus, e.g., turning head toward a loud noise or withdrawing hand from a hot plate)
2. Semivoluntary (unvoluntary): (a) induced by inner sensory stimulus (e.g., need to “stretch” a body part) and (b) induced by unwanted feeling or compulsion (e.g., compulsive touching or smelling)
3. Involuntary: (a) nonsuppressible (e.g., reflexes, seizures, myoclonus) and (b) suppressible (tics, tremor, dystonia, chorea, stereotypy)
4. Automatic: learned motor behaviors performed without conscious effort (e.g., walking or speaking); automatic, learned behaviors appear to be encoded in the sensorimotor portion of the striatum, which also may have a role in the generation of tics as learned voluntary motor skills may be incorporated into a tic repertoire.
Tics may be simple or complex. Simple motor tics involve only one group of muscles and cause brief jerk-like movements. They are usually abrupt in onset and rapid (clonic tics), but they may be slower and cause a briefly sustained abnormal posture (dystonic tics) or an isometric contraction (tonic tics) (6). Examples of simple clonic motor tics include blinking, nose twitching, and head jerking. Simple dystonic tics include blepharospasm, oculogyric movements, bruxism, sustained mouth opening, torticollis, and shoulder rotation; the tensing of abdominal or limb muscles is an example of tonic tic. Dystonic (and tonic) muscle contraction may be responsible for blocking tics, which are due to prolonged tonic or dystonic tics that interrupt ongoing motor activity, such as speech, or to a sudden inhibition of motor activity. We and others have drawn attention to the presence of tics and dystonia in the same family, providing evidence for a possible etiologic relationship between TS and primary dystonia (7,8).
Motor (particularly dystonic) and phonic tics are preceded by premonitory sensations in more than 80% of patients (9,10). This premonitory phenomenon consist of localizable sensations or discomforts, such as a “burning feeling” in the eye before a blink, “tension or crick in the neck” relieved by stretching of the neck or jerking of the head, “feeling of tightness or constriction” relieved by arm or leg extension, “nasal stuffiness” before a sniff, “dry or sore throat” before throat clearing or grunting, and “itching” before a rotatory movement of the scapula. The observed movement or sound sometimes occurs in response to these premonitory phenomena, and this “intentional” or “unvoluntary” component of the movement may be a useful feature differentiating tics from other hyperkinetic movement disorders, such as myoclonus and chorea. Chee and Sachdev (11) suggest that “sensory tics,” which we and others refer to as “premonitory sensations,” “represent the subjectively experienced component of neural dysfunction below the threshold for motor and phonic tic production.” Besides the local or regional premonitory sensations, this premonitory phenomenon may be nonlocalizable, less specific, and a poorly described feeling, such as an urge, anxiety, anger, and other psychic sensations (12). Many patients report that they have to repeat a particular movement to relieve the uncomfortable urge until “it feels good.” The “just right” feeling has been associated with compulsive behavior, and as such, the “unvoluntary” movement may be regarded as a “compulsive tic.”
Complex motor tics consist of coordinated, sequenced movements resembling normal motor acts or gestures that are inappropriately intense and timed. They may be seemingly nonpurposeful, such as head shaking or trunk bending, or they may seem purposeful, such as touching, throwing, hitting, jumping, and kicking. Additional examples of complex motor tics include gesturing “the finger” and grabbing or exposing one’s genitalia (copropraxia) or imitating gestures (echopraxia). Burping, vomiting, and retching have been described as part of the clinical picture of TS, but it is not clear whether this phenomenon represents a complex tic or some other behavioral manifestation of TS (13). Complex motor tics may be difficult to differentiate from compulsions, which frequently accompany tics, particularly in TS. A complex, repetitive movement may be considered a compulsion when it is preceded by, or associated with, a feeling of anxiety or panic, as well as an irresistible urge to produce the movement or sound because of fear that if it is not promptly or properly executed “something bad” will happen. However, this distinction is not always possible, particularly when the patient is unable to verbalize such feelings. Some coordinated movements resemble complex motor tics but may actually represent “pseudovoluntary” movements (parakinesias) designed to camouflage the tics by incorporating them into seemingly purposeful acts, such as adjusting one’s hair during a head jerk.
Simple phonic tics typically consist of sniffing, throat clearing, grunting, squeaking, screaming, coughing, blowing, and sucking sounds. Complex phonic tics include linguistically meaningful utterances and verbalizations, such as shouting of obscenities or profanities (coprolalia), repetition of someone else’s words or phrases (echolalia), and repetition of one’s own utterances, particularly the last syllable, word, or phrase in a sentence (palilalia). Some TS patients also manifest sudden and transient cessation of all motor activity (blocking tics) without alteration of consciousness.
In contrast to other hyperkinetic movement disorders, tics are usually intermittent and may be repetitive and stereotypic (Table 30.1). Tics may occur as short-term bouts or bursting or as long-term waxing and waning (14). They vary in frequency and intensity, often changing distribution. Typically, tics can be suppressed volitionally, although this may require intense mental effort. Suppressibility, while typical of tics, has been well documented in other hyperkinetic movement disorders but to a lesser degree. Using functional magnetic resonance imaging (MRI), Peterson et al. (15) showed decreased neuronal activity during periods of suppression in the ventral globus pallidus, putamen, and thalamus. There was increased activity in the right caudate nucleus, right frontal cortex, and other cortical areas normally involved in the inhibition of unwanted impulses (prefrontal, parietal, temporal, and cingulate cortices). Besides temporary suppressibility, tics are also characterized by suggestibility and exacerbation with stress, excitement, boredom, fatigue, and exposure to heat. Tics also may increase during relaxation following a period of stress.
In contrast to other hyperkinetic movement disorders that are usually completely suppressed during sleep, motor and phonic tics may persist during all stages of sleep (16). When they are concentrating on mental or physical tasks (such as when playing a video game or during orgasm), many patients note a reduction in their tics. Others have increased frequency and intensity of their tics when distracted, especially when they no longer have the need to suppress the tics. Tics are also typically exacerbated by dopaminergic drugs and by central nervous system (CNS) stimulants, including methylphenidate and cocaine (17). Finally, it should be noted that there is a broad spectrum of movements that may be present in patients with TS and that may be confused with tics, such as akathisia, chorea, dystonia, compulsive movements, and fidgeting as part of hyperactivity associated with ADHD (18,19).
| Differential Diagnosis of Tics |
Classification | Differential Diagnosis |
A. Simple motor tics |
|
1. Clonic | Myoclonus Chorea Seizures |
2. Dystonic | Dystonic Athetosis |
3. Tonic | Muscle spasms and cramps |
B. Complex motor tics Stereotypies Restless legs Seizures | Mannerisms |
Phenomenology |
|
Abrupt Chorea Hyperreflexia Paroxysmal dyskinesia Seizures | Myoclonus |
Sensory phenomenon (urge → relief) | Akathisia stereotypy Restless legs syndrome Dystonia |
Perceived as voluntary | Akathisia |
Suppressibility | All hyperkinesias but less than tics |
Decrease with distraction | Akathisia Psychogenic movements |
Increase with stress | Most hyperkinesias |
Increase with relaxation (after period of stress) | Parkinsonian tremor |
Multifocal, migrate | Chorea Myoclonus |
Fluctuate spontaneously | Paroxysmal dyskinesias Seizures |
Present during sleep | Myoclonus (segmental) Periodic movements Painful legs/moving toes Other hyperkinesias Seizures |
CLINICAL FEATURES OF TS
MOTOR SYMPTOMS
TS, the most common cause of tics, is manifested by a broad spectrum of motor and behavioral disturbances. This clinical heterogeneity often causes diagnostic difficulties and presents a major challenge in genetic linkage studies. To aid in the diagnosis of TS, the Tourette’s syndrome Classification Study Group (TSCSG) proposed diagnostic for TS (20), but the most widely accepted criteria are those formulated for “Tourette disorder” by the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) (21):
A. Both multiple motor and one or more vocal tics are present at some time during the illness, although not necessarily concurrently.
B. The tics may wax or wane in frequency but have persisted for more than 1 year since first tic onset.
C. Onset is before age 18 years.
D. The disturbance is not attributable to the physiologic effects of a substance (e.g., cocaine) or a general medical condition (e.g., Huntington’s disease, postviral encephalitis).
Kurlan (22) suggested another set of diagnostic criteria for genetic studies and introduced the term “Tourette disorder” for patients who have “functional impairment.” However, this does not take into account the marked fluctuations in symptoms and severity; some patients may be relatively asymptomatic at one time and clearly functionally impaired at another time. The Tourette Syndrome Association’s International Genetic Collaboration developed the Diagnostic Confidence Index (DCI), which consists of 26 “confidence factors” with weights given to each and a maximal total score of 100. The most heavily weighted diagnostic confidence factors include history of coprolalia, complex motor or vocal tics, a waxing and waning course, echo phenomenon, premonitory sensations, an orchestrated sequence, and age at onset. The DCI was found to be a useful instrument in assessing the lifetime likelihood of developing TS (23). Several instruments, some based on ratings of videotapes, have been developed to measure and quantitate tics, but they all have some limitations (24,25).
The clinical criteria are designed to assist in accurate diagnosis, in genetic linkage studies, and in differentiating TS from other tic disorders (Table 30.2). A body of evidence supports the notion that many patients, if not all, with other forms of idiopathic tic disorders represent one end of the spectrum in a continuum of TS. The most common and mildest of the idiopathic tic disorders is the transient tic disorder (TTD) of childhood. This disorder is essentially identical to TS except that the symptoms last for less than a year and, therefore, the diagnosis can be made only in retrospect. Chronic multiple-tic disorder (CMTD) is also similar to TS, but these patients have only motor or, less commonly, only phonic tics lasting at least a year. Chronic single-tic disorder (CSTD) is the same as CMTD, but these patients have only a single motor or phonic tic. This separation into TTD, CMTD, and CSTD seems artificial because all can occur in the same family and probably represent a variable expression of the same genetic defect.
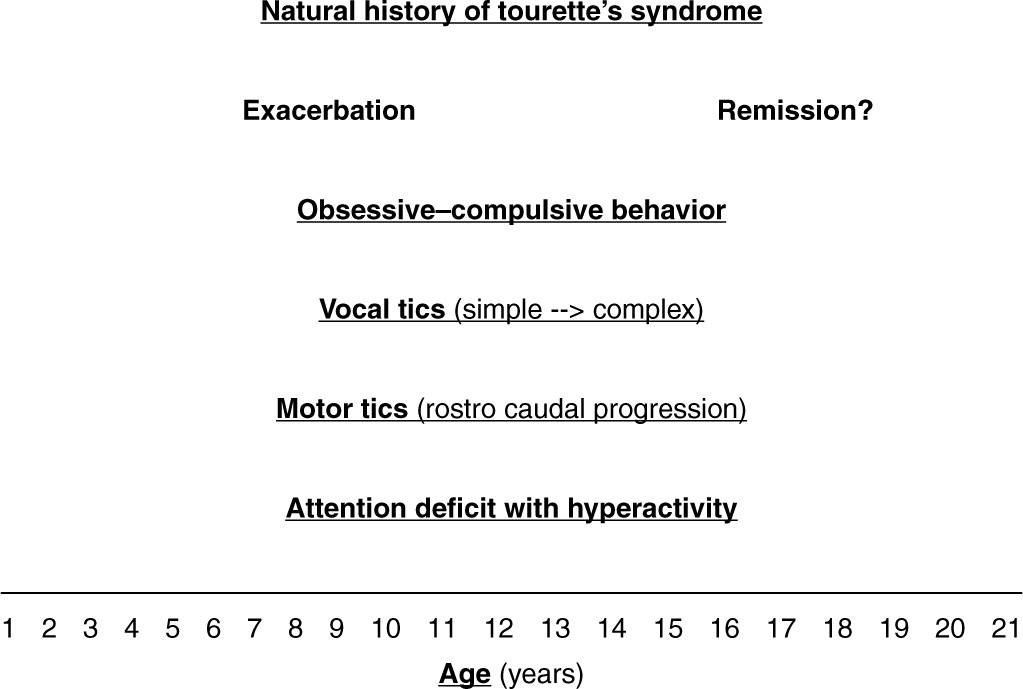
Although DSM-V criteria (21) require that symptoms start before the age of 18, nearly all patients with TS have symptoms before age 12 (26). In one longitudinal study, 46 children with TS underwent a structured interview at a mean age of 11.4 years, and again at 19.0 years (26). The mean worst-ever tic severity score was 31.6 out of a possible 50 on the Yale Global Tic Severity Scale (YGTSS), and occurred at a mean age of 10.6 years. By the time of the second interview, mean YGTSS score decreased to 10. This first prospective longitudinal study also showed that only 22% continued to experience mild or greater tic symptoms (YGTSS scores, ≥10) at follow-up, while nearly one-third were in complete remission of tic symptoms at follow-up. In another study, in 36% to 48% of patients, the initial symptom is eye blinking, followed by tics involving the face and head. Blink rate in persons with TS is about double that in normal, age-matched controls (27). During the course of the disorder, nearly all patients exhibit tics involving the face or head; two-thirds have tics in the arms; and one-half have tics involving the trunk or legs. According to another study, the average age at onset of tics is 5.6 years, and the tics usually become most severe at age 10; by 18 years of age, one-half of the patients are tic-free (28). In a study of 58 adults diagnosed with TS during childhood, Goetz et al. (29) found that tics persisted in all patients but were moderate or severe in only 24%, although 60% had moderate or severe tics during the course of the disorder. Tic severity during childhood had no predictive value for the future course, but patients with mild tics during the preadult period had mild tics during adulthood. Although the vast majority of tics in adults represent recurrences of childhood-onset tics, rare patients may have their first tic occurrence during adulthood. We reviewed 43 adults with TS referred to our Movement Disorders Clinic over the past 5 years and compared them with 100 TS patients 18 years old or younger (30). We found that adult TS patients had significantly more facial and truncal tics, as well as a greater prevalence of substance abuse and mood disorders, but fewer phonic tics, and lower rates of ADHD and oppositional behavior than children with TS. Furthermore, adult TS largely represented a reemergence or exacerbation of childhood-onset TS. During the course of TS, phonic and complex motor tics, self-injurious behaviors (SIBs), and ADHD tend to improve, but facial, neck, and trunk tics dominate the adult TS phenotype.
| Causes of Tics |
I. Primary
A. Sporadic
1. Transient motor or phonic tics (<1 y)
2. Chronic motor or phonic tics (>1 y)
3. Adult-onset (recurrent) tics
4. Tourette’s syndrome
5. Primary dystonia
B. Inherited
1. Tourette’s syndrome
2. Huntington’s disease
3. Primary dystonia
4. Neuroacanthocytosis
5. Neurodegeneration with brain iron accumulation
6. Tuberous sclerosis
7. Wilson’s disease
8. Duchenne’s muscular dystrophy
II. Secondary
A. Infections; encephalitis, Creutzfeldt–Jakob disease, neurosyphilis, Sydenham’s chorea
B. Drugs: amphetamines, methylphenidate, pemoline, levodopa, cocaine, carbamazepine, phenytoin, phenobarbital, lamotrigine, antipsychotics, and other dopamine receptor–blocking drugs (tardive tics, tardive tourettism)
C. Toxins: carbon monoxide
D. Developmental: static encephalopathy, mental retardation syndromes, chromosomal abnormalities, autistic spectrum disorders (Asperger’s syndrome)
E. Chromosomal disorders: Down syndrome, Kleinfelter’s syndrome, XYY karyotype, fragile X, triple X, 9p mosaicism, partial trisomy 16, 9p monosomy, citrullinemia, Beckwith–-Wiedemann syndrome
F. Other: head trauma, stroke, neurocutaneous syndromes, schizophrenia, neurodegenerative diseases
III. Related Manifestations and Disorders
A. Stereotypies/habits/mannerisms
B. Self-injurious behaviors
C. Motor restlessness
D. Akathisia
E. Compulsions
F. Excessive Sstartle
G. Jumping Frenchman
In adults with new-onset tics, it is important to search for secondary causes, such as infection, trauma, cocaine use, and neuroleptic exposure (17,18,31). Poor motor control, which can lead to poor penmanship and, at times, almost illegible handwriting, may contribute to the academic difficulties faced by many patients with TS. Tics, though rarely disabling, can be quite troublesome for TS patients because they cause embarrassment, interfere with social interactions, and at times can be quite painful or uncomfortable. Rarely, they can cause secondary neurologic deficits, such as cervical compressive myelopathy in patients with violent head and neck tics (32).
Vocalizations have been reported as the initial symptom in about one-third of all patients, with throat clearing being the most common initial phonic tic (33). Phonic tics can be troublesome for patients and those around them, particularly when they consist of loud, shrieking sounds. In addition to involuntary noises, some patients have speech disfluencies that resemble developmental stuttering, and up to one-half of all patients with developmental stuttering have been thought to have undiagnosed TS (34). Coprolalia, perhaps the most recognizable and certainly one of the most distressing symptom of TS, is actually present in only one-half of patients. When describing the distress caused by his severe coprolalia, one of our patients remarked that immediately after shouting an obscenity he reaches out with his hand in an attempt to “catch the word and bring it back before others can hear it.” This symptom appears to be markedly influenced by cultural background. Although in one retrospective analysis of 112 children with TS, only 8% exhibited coprolalia (35), the true prevalence of coprolalia in TS children and adults is only about 50% in the US population, even when mental coprolalia (without actual utterance) is included. In a study of 597 individuals with TS from seven countries, coprolalia occurred at some point in the course of the disease in 19.3% of males and 14.6% of females, and copropraxia in 5.9% of males and 4.9% of females (36).
Except for the presence of tics, the neurologic examination in patients with TS is usually normal. In one case–control study, TS patients were found to have a shorter duration of saccades, but the saccades were performed with a greater mean velocity than normal controls and were associated with fewer correct antisaccade responses, suggesting a mild oculomotor disturbance in TS (37). Although the ability to inhibit reflexive saccades is normal, TS patients make more timing errors, indicating an inability to appropriately inhibit or delay planned motor programs.
BEHAVIORAL SYMPTOMS
In addition to motor and phonic tics, patients with TS often exhibit a variety of behavioral symptoms, particularly ADHD and OCD (Fig. 30.1). Diagnosis of ADHD and OCD is based on clinical history; there are no laboratory or other tests that reliably diagnose these neurobehavioral disorders (38). These comorbid behavioral conditions often interfere with learning and with academic and work performance. In contrast to tics, ADHD and obsessional symptom severity are significantly associated with impaired social and emotional adjustment (39). The clinician should be skilled not only in the recognition and management of ADHD but also in documenting the ADHD-related deficits (40). Such documentation is essential for parents and educators to provide the optimal educational setting for the affected individual.
Since nearly all studies on the frequency of associated features have been based on a population of TS patients referred to physicians (usually specialists), there is a selection bias; therefore, accurate figures on the prevalence of these behavioral disorders in TS patients are not available. Most patients with TS have had symptoms of ADHD, OCD, or both sometime during the course of their illnesses (41). The symptoms of ADHD may be the initial manifestations of TS and may precede the onset of motor and phonic tics by about 3 years. Despite growing publicity about ADHD, there is little evidence of widespread overdiagnosis or overtreatment of this disorder. Based on an interview of 1,596 children, aged 9 to 17, in Rochester and Monroe County (New York) schools, tics were identified in 339 (21%) after 60 to 150 minutes of observation (42). The investigators found the following behavioral problems more frequently (p <0.05) in children with tics than in those without tics: OCD, ADHD, separation anxiety, overanxious disorder, simple phobia, social phobia, agoraphobia, mania, major depression, and oppositional defiant behavior. Also, children with tics were younger (mean age: 12.5 versus 13.3 years) and were more likely to require special education services (27% versus 19.8%).
There are three types of ADHD: predominantly inattentive, predominantly hyperactive–impulsive, and combined (41). Although attention deficit is certainly one of the most common and disabling symptoms of TS, in many patients the inability to pay attention is due not only to a coexistent ADHD but also to uncontrollable intrusions of thoughts. Some patients are unable to pay attention because of a compulsive fixation of gaze. For example, while sitting in a classroom or a theater or during a conversation, the gaze becomes fixed on a particular object and, despite concentrated effort, patients are unable to break the fixation. As a result, they miss the teacher’s lesson or a particular action in a play. Another reason for impaired attention in some TS patients is mental concentration exerted in an effort to suppress tics. Yet another cause for inattention is the sedative effect of anti-TS medications. It is, therefore, important to determine which mechanism or mechanisms are most likely responsible for the patient’s attention deficit. Although genetics clearly plays a key role in the mechanism of attention-deficit disorder (ADD) and ADHD, the gene(s) or other causes have not been fully elucidated (43). One study showed that children and adolescents with ADHD, compared to those without ADHD, are more likely to have major injuries and asthma, and their 9-year medical costs are double (44).
Although OCD frequently occurs alone without other features of TS (45), it is now well accepted that OCD is part of the spectrum of neurobehavioral manifestations in TS (45,46). With an estimated lifetime prevalence of 2% to 3% (46) and an incidence of 0.55 per 1,000 person-years (47), OCD is one of the most common causes of disability (48). The instrument used most frequently to measure the severity of OCD is the Yale–Brown Obsessive–Compulsive Scale (49). A distinction should be made between obsessive–compulsive symptoms or traits, obsessive–compulsive personality disorder, and OCD. Obsessions are characterized by intense, intrusive thoughts, such as concerns about bodily wastes and secretions; unfounded fears; a need for exactness, symmetry, evenness, and/or neatness; excessive religious concerns; perverse sexual thoughts; and intrusions of words, phrases, or music. Compulsions consist of a subjective urge to perform meaningless and irrational rituals, such as checking, counting, cleaning, washing, touching, smelling, hoarding, and rearranging. Leckman et al. (50) have drawn attention to the frequent occurrence of the “just right” perception in patients with OCD and TS. Whereas obsessional slowness accounts for some of the school problems experienced by TS patients, cognitive slowing (bradyphrenia) is also a contributing factor (51). In contrast to primary OCD in which the symptoms relate chiefly to hygiene and cleanliness, the obsessive symptoms associated with TS usually involve concerns with symmetry, violent aggressive thoughts, forced touching, fear of harming self or others, and a need to say or do things “just right” (52). A principal-components factor analysis of 13 categories used to group types of obsessions and compulsions in the Yale–Brown Obsessive–Compulsive Scale symptom checklist identified the obsessions and checking and the symmetry and ordering factors as particularly common in patients with tic disorders (53). In addition to an idiopathic sporadic or familial disorder and TS, OCD has been reported to occur as a result of a variety of lesions in the frontal–limbic–subcortical circuits (54). Cognitive inflexibility manifested by impaired task-switch ability in patients with OCD has been suggested to be due to “imbalance in brain activation between dorsal and ventral striatal circuits” (46).
One of the most troublesome symptoms of TS is poor impulse control manifested sometimes by inability to control anger, as a result of which many patients may exhibit frequent, and sometimes violent, temper outbursts and rages. Indeed, many behavioral symptoms of TS, including some complex tics, coprolalia, copropraxia, and many behavioral problems, can be explained by loss of normal inhibitory mechanisms (disinhibition) manifested by poor impulse control. Rarely, TS patients exhibit inappropriate sexual aggressiveness, as well as antisocial, oppositional, and even violent, unlawful, or criminal behavior. Indeed, TS serves as a model medical disorder that may predispose one to engage in uncontrollable and offensive behaviors that are misunderstood by the law-abiding community and legal justice system (55). The social and legal aspects of TS have yet to be investigated, but there is growing concern regarding media misrepresentation that attributes violent criminal behavior in certain individuals to TS. Although TS should not be used as an “excuse” to justify unlawful or criminal behaviors, studies are needed to determine whether TS-related symptoms and neurobehavioral comorbidities predispose individuals with TS to engage in such behaviors. Often, the avolitional nature of behaviors in response to involuntary internal thought and emotional patterns is supported by the subsequent remorse and lack of secondary gain. This suggests that the preponderance of unlawful acts committed by TS patients are not premeditated but may result from a variety of TS-related mechanisms, such as poor impulse control, OCD associated with addictive behavior (e.g., drugs, alcohol, gambling), and ADD and distractibility (e.g., motor vehicle accidents). In one study, TS accounted for 2% of all cases referred for forensic psychiatric investigation in Stockholm, Sweden, between 1990 and 1995; 15% of offenders had ADHD, 15% had pervasive developmental disorder (PDD), and 3% had Asperger’s syndrome (56). There is no evidence that anti-TS medications increase risk for criminal behavior and, in fact, anti-ADHD medications have been found to reduce the risk of criminality (57).
Focal frontal lobe dysfunction, demonstrated in TS by various functional and imaging studies, has been associated with an impulsive subtype of aggressive behavior (58). It has been postulated that impulse disorders stem from exaggerated reward-, pleasure-, or arousal-seeking brain centers, resulting in failure of inhibition. Animal studies of rats with lesions of the nucleus accumbens core, the brain region noted for reward and reinforcement, showed that the lesioned rats preferred small immediate rewards to larger delayed rewards (59). In addition to the ventromedial prefrontal cortices, lesions in the amygdala also have been known to cause alterations in decision-making processes and disregard for consequences (60).
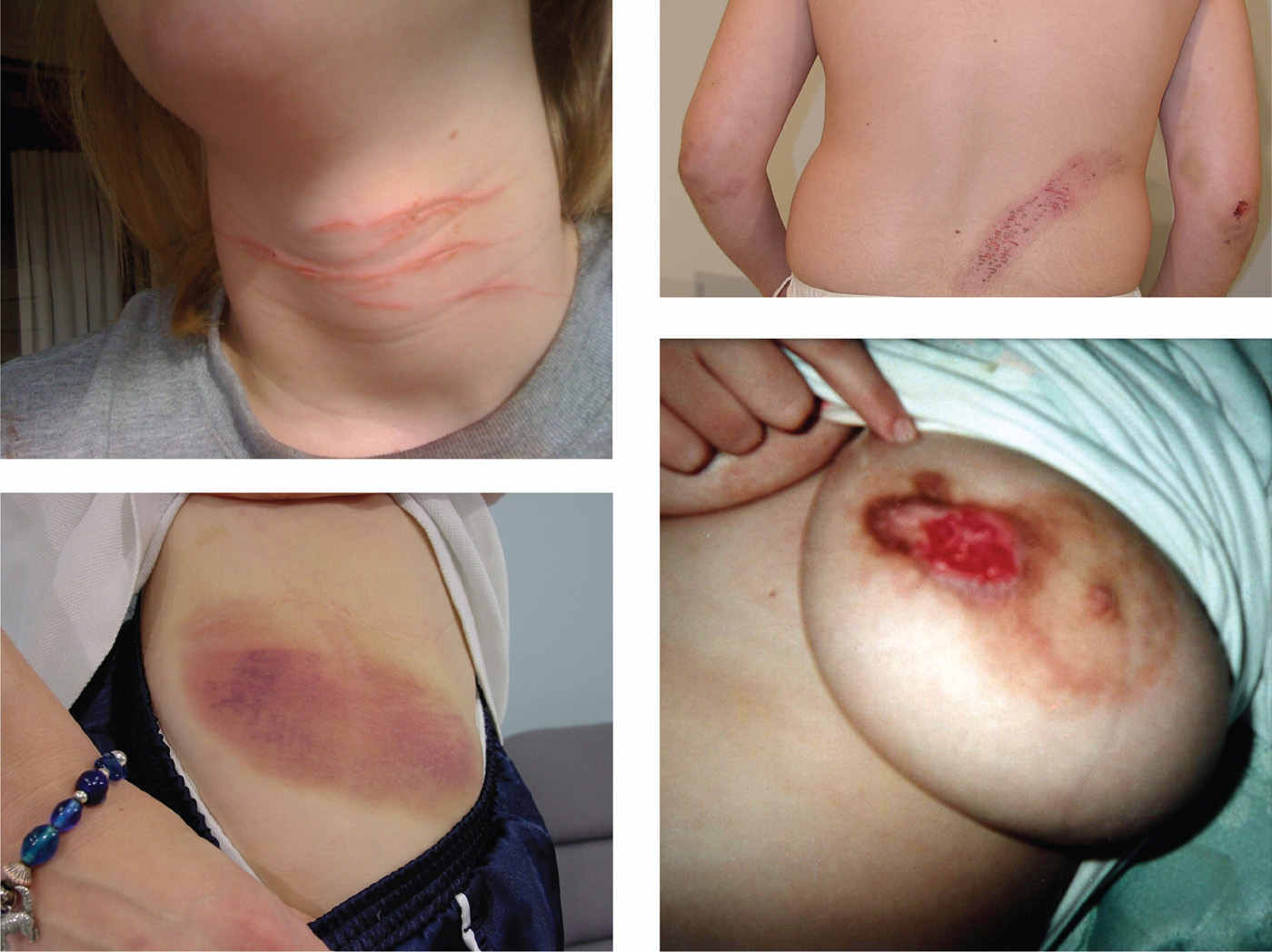
One of the most distressing symptoms of TS is a SIB, reported in up to 53% of all patients (61,62). A common form of SIB is damage of skin by compulsive biting, scratching, cutting, engraving, or hitting (particularly in the eye and throat), often accompanied by an irresistible urge (obsession) (Fig. 30.2) (4). Thus, SIB appears to be related to OCD, which has treatment implications. Of 332 TS patients evaluated at Baylor College of Medicine Movement Disorders Clinic during a 3-year period, 17 (5.1%) met criteria for malignant TS, defined as less than or equal to two emergency room visits or less than or equal to one hospitalization for TS symptoms or its associated behavioral comorbidities (63). The patients exhibited tic-related injuries, SIB, uncontrollable violence and temper, and suicidal ideation/attempts. Compared to patients with nonmalignant TS, those with malignant TS were significantly more likely to have a personal history of obsessive–compulsive behavior/disorder (OCB/OCD), complex phonic tics, coprolalia, copropraxia, SIB, mood disorder, suicidal ideation, and poor response to medications. Severe or malignant TS, associated with SIB and other disabling features, has been also reported in families, including consanguine kindreds (64).
The TS, in addition to tics, ADHD, and OCD, can be associated with a variety of behavioral manifestations, including learning and conduct disorders, schizoid and affective disorders, antisocial behaviors, oppositional defiant disorder, anxiety, depression, conduct disorder, severe temper outbursts, rage attacks, impulse control problems, inappropriate sexual behavior, and other psychiatric problems (61,62). Personality disorder and depression have been reported in 64% of patients with TS (62). Besides comorbid behavioral conditions, TS has been reported to be frequently associated with migraine headaches, which may be related to the coexistent OCD (65). The Tourette International Consortium Database, which at the time of its publication in 2000 included information on 3,500 patients with TS collected from 64 centers from around the world, showed that only 12% of patients with TS had no other disorders; ADHD was seen in 60%, symptoms of OCD in 59%, anger-control problems in 37%, sleep disorder in 25%, learning disability in 23%, mood disorder in 20%, anxiety disorder in 18%, and SIB in 14% (66).
Other related conditions that may have resemblance to tics and TS are the jumping Frenchmen of Maine, “ragin’ Cajuns” of Louisiana, latah of the Malays, and myriachit of Siberia (67,68). First described in 1878, these culture-bound conditions are characterized by an excessive startle response, sometimes with echolalia, echopraxia, or forced obedience. It is considered by some as an operant conditioned behavior rather than a neurologic or even hysteric disorder. Rarely, psychogenic tics are present in patients with TS, but this very rare occurrence is difficult to confirm clinically (69). In a series of nine patients (five females; mean age at onset was 34.1 years) with psychogenic tics, the following features helped to differentiate these patients from those with organic tics: lack of premonitory sensations, absence childhood and family history of tics, inability to suppress the movements, and coexistence with other psychogenic movement disorders and pseudoseizures. Furthermore, compared with patients with TS, those with psychogenic tics were more likely to be females.
PATHOPHYSIOLOGY
NEUROPHYSIOLOGY
Although the pathogenic mechanisms of TS are unknown, the weight of evidence supports organic rather than psychogenic origin, involving the cortical–striatal circuitry (70). Despite the observation that some tics may be, at least in part, voluntary, physiologic studies suggest that tics are not mediated through normal motor pathways utilized for willed movements. About 20% of patients with TS have exaggerated startle responses, which may fail to habituate with repetition (71). Using back-averaging techniques, premotor negativity has been demonstrated in some patients with simple motor tics (72). Although the investigators could not correlate the presence of Bereitschaftspotential with the premonitory sensation, the physiology of the premovement phenomenon requires further study.
Functional MRI studies in patients with TS have shown decreased neuronal activity during periods of suppression in the ventral globus pallidus, putamen, and thalamus and increased activity in the right caudate nucleus, right frontal cortex, and other cortical areas normally involved in the inhibition of unwanted impulses (prefrontal, parietal, temporal, and cingulate cortices) (73). In another study utilizing functional MRI, Serrien et al. showed marked reduction or absence of activity in secondary motor areas, while the patients attempted to maintain a stable grip-load force control (74). The authors interpreted the findings as ongoing activation of the secondary motor areas reflecting patients’ involuntary urges to move. In a study of children with ADHD, functional MRI showed increased frontal activation and reduced striatal activation on various tasks, and an enhancement of striatal function after treatment with methylphenidate (74). Transcranial magnetic stimulation (TMS) studies have demonstrated shortened cortical silent period and defective intracortical inhibition (determined in a conditioning test paired-stimulus paradigm) in patients with TS and OCD (75), thus providing possible explanation for intrusive phenomena. Subsequent studies using the same technique have demonstrated that patients with tic-related OCD have more abnormal motor cortex excitability than OCD patients without tics (76). TMS studies also have demonstrated that TS children have a shorter cortical silent period but that their intracortical inhibition is not different from that of controls, although intracortical inhibition is reduced in children with ADHD (77). There is evidence of additive inhibitory deficits, as demonstrated by reduced intracortical inhibition and shortened cortical silent period in children with TS and comorbid ADHD. Both short-interval intracortical inhibition and short-interval afferent inhibition were reduced in 8 patients with TS (aged 24–38 years) as compared to 10 matched healthy controls (78).
Sleep studies have provided additional evidence that some tics are truly involuntary (16). Polysomnographic studies in TS patients recorded motor and phonic tics in various stages of sleep and have found that some patients with TS have alterations of arousal; decreased percentage of stage ¾ (slow-wave) sleep; decreased percentage of rapid eye movement (REM) sleep; paroxysmal events in stage 4 sleep with sudden intense arousal, disorientation, and agitation; restless legs syndrome; periodic leg movement during sleep; and other sleep-related disorders, including sleep apnea, enuresis, sleep walking and sleep talking, nightmares, myoclonus, bruxism, and other disturbances (79).
NEUROIMAGING
Although standard anatomic neuroimaging studies in TS are unremarkable, using special volumetric, metabolic, blood flow, ligand, and functional imaging techniques, several interesting findings have been reported that have strong implications for the pathophysiology of TS. Careful volumetric MRI studies have suggested that the normal asymmetry of the basal ganglia is lost in TS. Frederickson et al. (80) found evidence of smaller gray matter volumes in left frontal lobes of patients with TS, further supporting the findings of loss of normal left–right asymmetry. Quantitative MRI studies have found subtle but possibly important reduction in the volume of caudate nuclei in patients with TS. In 10 pairs of monozygotic twins, the right caudate was smaller in the more severely affected individuals, providing evidence for the role of environmental events in the pathogenesis of TS (81). In contrast, the corpus callosum has been found to be larger in children with TS than in normal controls (82). Subsequent study has showed that this finding was gender-related and was present only in boys with TS (83). In another study, caudate volumes have been reported to correlate significantly and inversely with the severity of tics and OCD in early adulthood (84).
Positron emission tomography (PET) scanning has shown variable rates of glucose utilization in basal ganglia, compared with controls. In one study, 18F-fluorodeoxyglucose (FDG) PET has shown evidence of increased metabolic activity in the lateral premotor and supplementary motor association cortices and in the midbrain (pattern 1), and decreased metabolic activity in the caudate and thalamic areas (limbic basal ganglia–thalamocortical projection system) (pattern 2) (85). Pattern 1 is reportedly associated with tics, and pattern 2 correlates with the overall severity of TS. In contrast to dystonia, characterized by lentiform nucleus–thalamic metabolic dissociation, attributed to overactivity of the direct striatopallidal inhibitory pathway, the pattern of TS is characterized by concomitant metabolic reduction in striatal and thalamic function. The authors suggested that this pattern could be explained by a reduction in the indirect pathway resulting in reduction in subthalamic nucleus activity. Using event-related [15O]H2O PET combined with time-synchronized audio- and videotaping in six patients with TS, Stern et al. (86) found increased activity in the sensorimotor, language, executive, paralimbic, and frontal–subcortical areas that were temporarily related to the motor and phonic tics and the irresistible urge that precedes these behaviors. Rauch et al. (87) showed bilateral medial temporal (hippocampal/parahippocampal) activation on PET in patients with OCD, compared with normal controls, and absence of activation of inferior striatum, seen in normal controls. Various neuroimaging studies also have demonstrated moderate reduction in the size of the corpus callosum, basal ganglia (particularly the caudate and globus pallidus), and frontal lobes and striatal hypoperfusion in patients with ADHD (88).
NEUROCHEMISTRY
Neurochemical studies of TS have been hampered by the unavailability of postmortem brain tissue. Biochemical abnormalities in the few postmortem brains that have been studied include low serotonin, low glutamate in the internal globus pallidus, and low cyclic adenosine monophosphate (cAMP) in the cortex (89). An alteration in the central neurotransmitters in TS also has been suggested, chiefly because of relatively consistent responses to modulation of the dopaminergic system. Dopamine antagonists and depletors generally have an ameliorating effect on tics, whereas drugs that enhance central dopaminergic activity exacerbate tics. Low levels of homovanillic acid in cerebrospinal fluid, coupled with a favorable response to dopamine receptor–blocking drugs, have been interpreted as evidence in support of the notion that tics and TS are due to supersensitive dopamine receptors. There is, however, little or no evidence based on postmortem or imaging studies of increased striatal dopaminergic innervation in TS (90).
An abnormality in the GABAergic system in TS has gained more support based on functional imaging studies. For example, in a study of 11 patients with TS and 11 healthy controls, PET scan using [(11)C]flumazenil and structural MRI provided evidence of decreased binding of GABA(A) receptors in TS patients bilaterally in the ventral striatum, globus pallidus, thalamus, amygdala, and right insula and increased binding in the bilateral substantia nigra, left periaqueductal gray, right posterior cingulate cortex, and bilateral cerebellum (91).
Despite some limitations and inconsistencies, the imaging, ligand, and biochemical studies provide support for the hypothesis that the cortico–striato–thalamo–cortical circuit has an important role in the pathogenesis of TS and related disorders (2). The dorsolateral prefrontal circuit, which links Brodmann’s areas 9 and 10 to the dorsolateral head of the caudate, appears to be involved with executive functions (manipulation of previously learned knowledge, abstract reasoning, organization, verbal fluency, and problem-solving; closely related to intelligence, education, and social exposure) and motor planning. An abnormality in this circuit has been implicated in ADHD. The lateral orbitofrontal circuit originates in the inferior lateral prefrontal cortex (area 10) and projects to the ventral medial caudate. An abnormality in this circuit is associated with personality changes, mania, disinhibition, and irritability. Lastly, the anterior cingulate circuit arises in the cingulate gyrus (area 24) and projects to the ventral striatum, which also receives input from the amygdala, hippocampus, medial orbitofrontal cortex, and the entorhinal and perirhinal cortices. A variety of behavioral problems, including OCD, may be linked to an abnormality in this circuit.
GENETICS
Finding a genetic marker, and ultimately the responsible gene, has been the highest priority in TS research during the past decade. Unfortunately, despite a concentrated effort by many investigators, the TS gene has thus far eluded this intensive search (92). We compared our TS patients with a control population of 1,142 students, observed in second-, fifth-, and eighth-grade classrooms. In contrast to 5% frequency of ADD in one parent of controls, the occurrence of tics in at least one parent of TS cases was 31%, ADD 45%, and OCD 41%. Among all parents of TS cases, tics were present in 24%, OCD in 25%, and ADD in 34%, whereas only 3% of parents of controls exhibited ADD (93). Bilineal transmission violates the standard principle of one trait/one locus and may explain why a gene marker has not yet been identified in TS despite intense collaborative research. Our results are similar to those of Lichter et al. (94) who found bilineal transmission in 6% of patients; tics or OCD represented bilineally in 22%. In a large family study and segregation analysis, Walkup et al. (95) provided evidence for a mixed model of inheritance rather than a simple autosomal model of inheritance. This complex model of inheritance suggests that the majority of TS patients have two copies of the gene, one from each parent. This is consistent with the observation that both parents of many TS patients are affected.
How much influence environmental factors have on the phenotypic expression of this disorder is not known. Leckman et al. (96) in a search for nongenetic factors in the pathogenesis of TS found that maternal life stress, nausea, and vomiting during the first trimester of pregnancy were some of the perinatal factors that influenced the expression of the TS gene. In a study of 16 pairs of monozygotic twins, 94% of whom were concordant for tics, low birth weight was a strong predictor of tic severity, supporting a relationship between birth weight and phenotypic expression of TS (97). A major advance in the search for the elusive TS gene or genes has been made by the discovery of a frameshift mutation in the Slit and Trk-like 1 (SLITRK1) gene on chromosome 13q31.1 (98). These variants were absent in 172 other TS and in 3,600 control chromosomes. The SLITRK1 gene has been found to be expressed in brain regions previously implicated in TS, such as the cortex; the hippocampus; thalamic, subthalamic, and globus pallidus nuclei; striatum; and cerebellum. It also appears to play a role in dendritic growth. Although we did not find a mutation in the SLITRK1 gene in any of our TS patients, suggesting that this gene abnormality is a rare cause of TS (99); the gene discovery represents an important step forward in search of cellular mechanisms of TS.
Although no TS-specific gene or genes have been found (100), there is some progress being made in identifying gene loci through genome-wide association studies. In one study involving 1,285 cases and 4,964 ancestry-matched controls, no markers achieved a genome-wide threshold of significance, but a signal was found in rs7868992 on chromosome 9q32 within the COL27A1 gene which codes for a fibrillar collagen primarily expressed in cartilage and in the cerebellum during development (101). In an attempt to examine the contribution of large, rare copy number variants to TS and OCD susceptibility using a genome-wide, cross-disorder design in 2,699 cases (1086 TS, 1613 OCD) and 1,789 controls, deletions were located in the locus 16p13.11, suggesting that mutations in this region may be associated with increased susceptibility of TS, OCD, and possibly other neurodevelopmental disorders (102).
SECONDARY CAUSES OF TICS
In addition to TS, there are many other genetic and nongenetic causes of tics (see Table 30.2). Variably referred to as pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), this condition is one of the most controversial topics in pediatric neurologic and psychiatric literature (2). Briefly, several studies have suggested that the onset of or an exacerbations of TS symptoms correlated with an antecedent group A β-hemolytic streptococcal (GABHS) infection. In an attempt to address the controversy surrounding PANDAS, a new entity, termed “Pediatric Acute-onset Neuropsychiatric Syndrome” (PANS), has been proposed to acknowledge that there is a subgroup of children presenting with an abrupt onset of OCD and acute neuropsychiatric symptoms, accompanied by a variety of comparably severe and acute neuropsychiatric symptoms (103). In contrast to PANDAS, the diagnostic criteria for PANS no longer include tics.
EPIDEMIOLOGY
Discovery of a disease-specific marker will be helpful not only in improving our understanding of this complex neurobehavioral disorder but also in clarifying the epidemiology of TS. Thus, epidemiologic studies have shown that 20% to 30% of children exhibit tics sometime during childhood and 2% to 3% of children develop some features of TS, although the worldwide prevalence of TS in children has been reported to range from 0.3% to 0.8% (104). There are many reasons for this wide variation, the most important of which are different ascertainment methods, different study populations, and different clinical criteria. Since about one-third of patients with tics do not recognize their presence, it is difficult to derive more accurate prevalence figures for TS without a well-designed door-to-door survey. Our own observational study involved 1,142 children in second, fifth, and eighth grades of general school population, among whom 8 (0.7%) had some evidence of TS (93). Meta-analysis of 13 studies of children yielded a prevalence of TS of 0.77% (95% confidence interval, 0.39%–1.51%); the boys:girls ratio was 1.06%: 0.25%, and meta-analysis of two studies assessing adults with TS revealed a prevalence of 0.05% (95% confidence interval, 0.03%–0.08%) (105). The prevalence of tic disorders was higher in all studies performed in special education population. Similar findings were reported based on a prospective cohort study following 6,768 children in Avon, United Kingdom, where 0.3% of 13-year-old children met criteria for clinically definite TS and 0.7% with clinically probably TS (106). It is not known why tics disappear in majority of children, and further studies are needed to understand mechanisms of conversion from pre-TS state to TS.
A unifying hypothesis for the pathogenesis of TS suggests that TS represents a developmental disorder resulting in dopaminergic hyperinnervation of the ventral striatum and the associated limbic system. Although highly speculative, it is possible that the genetic defect in TS somehow interferes with normal apoptosis during development, resulting in the increased innervation of the ventral striatum and other limbic areas (107). This implies that the genetic defect interferes somehow with the programmed cell suicide needed to control cell proliferation in normal development and growth. The link between the basal ganglia and the limbic system may explain the frequent association of tics and complex behavioral problems, and a dysfunction in the Cortico–Striatal–Thalamic–Cortical (CSTC) circuitry seems to provide the best explanation for the most fundamental behavioral disturbance in TS, namely, loss of impulse control and a state of apparent disinhibition. There are currently no animal models of TS, except for some families of horses with equine self-mutilation syndrome with features resembling human TS (108). However, future genetic studies and animal models (109,110) should provide insights into the pathogenesis of this complex neurobehavioral disorder and lead to animal models on which this and other hypotheses can be tested.
TREATMENT
The first step in the management of patients with TS is proper education of the patients, relatives, teachers, and other individuals who frequently interact with the patient regarding the nature of the disorder. School principals, teachers, and students can be helpful in implementing the therapeutic strategies. In addition, parents and the physician should work as partners in advocating the best possible school environment for the child. This may include extra break periods and a refuge area to “allow” release of tics, waiving time limitations on tests or adjusting timing of tests to the morning, and other measures designed to relieve stress. National and local support groups, particularly the Tourette Syndrome Association, can provide additional information and can serve as a valuable resource for the patient and his or her family (see For Further Information). Counseling and behavioral modification may be sufficient for those with mild symptoms. However, medications may be considered when symptoms begin to interfere with peer relationships, social interactions, academic or job performance, or activities of daily living. Because of the broad range of neurologic and behavioral manifestations and the varying severity of TS, treatment must be individualized and tailored specifically to the needs of the patient (Fig. 30.3; Table 30.3) (2,111).
Before discussing pharmacologic therapy of TS symptoms, it is appropriate to make a few remarks about behavioral therapy. Different forms of behavioral modification have been recommended since the disorder was first described, but until recently, few studies of behavioral treatments had been subjected to rigorous scientific scrutiny. Most of the reported studies suffer from poor or unreliable assessments, small sample size, short follow-up, lack of controls, no validation of compliance, and other methodologic flaws. Given these limitations, the following behavioral techniques have been reported to provide at least some benefit (112):
• Massed (negative) practice: Voluntary and effortful repetition of the tic leads to a buildup of a state termed “reactive inhibition,” at which point the subject is forced to rest and not perform the tic due to an accumulation of “negative habit.”
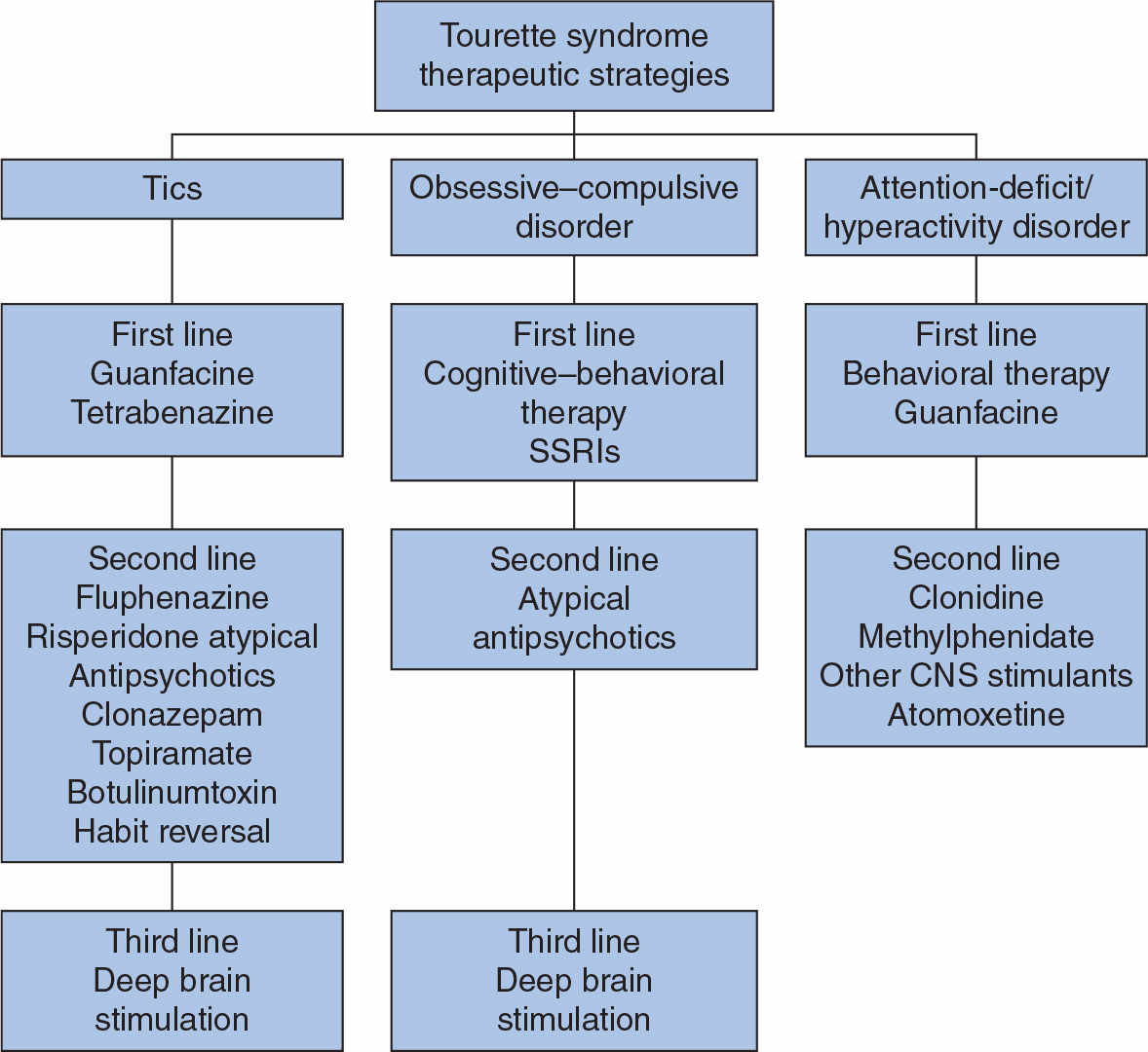
Figure 30.3. Algorithm for the management of TS. (Modified Jankovic J, Kurlan R. Tourette syndrome: evolving concepts. Mov Disord 2011;26(6):1149–1156.)