Topiramate
Michael D. Privitera
HISTORICAL BACKGROUND
Topiramate (TPM) is a highly oxygenated sulfamate-substituted monosaccharide that is structurally distinct from other anticonvulsant medications. Available in the United States as Topamax (Ortho-McNeil Pharmaceutical), it is a broad-spectrum agent that has been extensively studied in double-blind, randomized, controlled trials in adults and children. Initially approved for use as adjunctive therapy in partial-onset seizures, primary generalized tonic-clonic seizures, and multiple seizure types associated with Lennox-Gastaut syndrome, it is currently under evaluation for approval as first-line therapy in the United States.
CHEMISTRY
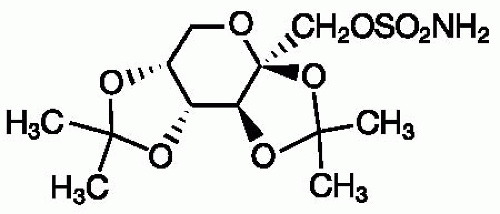
TPM (2,3:4,5-di-O-isopropylidene-β-D-fructopyranose sulfamate; Fig. 61.1) is a white crystalline powder, which is freely soluble in acetone, chloroform, dimethylsulfoxide, and ethanol. TPM is supplied as 25-, 50-, 100-, and 200-mg tablets and as 15- and 25-mg sprinkle capsules that can be opened and sprinkled onto soft food for children and for patients who may have difficulty swallowing tablets.
MECHANISMS OF ACTION
TPM has a unique combination of activities at various receptor sites and ion channels, which may account for its broad-spectrum profile in epilepsy and other neurologic disorders. It blocks the kainate/AMPA (α-amino-3-hydroxy-5-methy-lisoxazole-4-propionic acid) subtype of the glutamate receptor (1, 2, 3, 4), with no direct effect on NMDA (N-methyl-D-aspartate) receptor activity; blocks voltage-activated sodium channels to limit sustained repetitive firing (5, 6, 7, 8, 9); enhances γ-aminobutyric acid (GABA)-mediated chloride flux at GABAA receptors (10,11); reduces the amplitude of high-voltage-activated calcium currents (12,13); and activates potassium conductance (14,15). It has been hypothesized that effects of TPM on voltage-activated sodium channels, high-voltage-activated calcium channels, GABAA receptors, and AMPA/kainate receptors reflect a common modulator involving protein phosphorylation (16). TPM is also a weak inhibitor of carbonic anhydrase isoenzymes (CA II and CA IV), which may modulate pH-dependent activation of volt-age-and receptor-gated ion channels (17); its inhibitory effect is less than acetazolamide.
The anticonvulsant properties of TPM have been demonstrated in several animal models of epilepsy; these have been discussed elsewhere (16,18, 19, 20, 21). Experimental studies have shown that TPM reduces seizure-induced hippocampal neuronal injury (22) and prevents spontaneous seizures following status epilepticus (23). In an experimental model of neonatal hypoxia/ischemia, TPM suppressed acute seizures and reduced subsequent susceptibility to neuronal injury and seizures induced by a second insult (kainate) (24).
PHARMACOKINETICS
Renal elimination, low protein binding, and a long half-life make TPM relatively easy to manage from a pharmacokinetic perspective.
Absorption
TPM is rapidly absorbed with peak plasma concentrations occurring in 1 to 4 hours with TPM doses of 100 to 400 mg (25). Absorption is nearly complete with less than 80% of a 100-mg dose recovered in urine (25). Coadministration with food slightly delays absorption but does not decrease bioavailability (25). TPM exhibits linear kinetics; plasma concentrations increase in proportion to dose increases (26).
Distribution and Protein Binding
The apparent volume of distribution for TPM is 38.5 to 58 L (0.6 to 0.8 L/kg, weight normalized), consistent with distribution to total body water. Binding to plasma proteins is minimal (13% to 17%) and is not considered to be a major factor in dosing and drug interactions (26).
Metabolism and Excretion
In the absence of hepatic enzyme induction, approximately 20% of a TPM dose is metabolized. When TPM is co-administered with enzyme-inducing antiepileptic drugs (AEDs), up to 50% of the TPM dose may be metabolized. Hepatic metabolism appears to involve hydroxylation, hydrolysis, and glucuronidation; none of the metabolites constitutes >5% of an administered dose, and they are quickly cleared (26).
Elimination of TPM is primarily via renal excretion, with 50% to 80% being eliminated in the urine unchanged. The half-life of TPM in adults is 20 to 30 hours in the absence of enzyme induction, allowing steady-state plasma concentrations to be reached in 4 to 8 per day. In the presence of enzyme induction, the TPM half-life in adults is 12 to 15 hours (26). In children 4 to 17 years of age, clearance is approximately 50% higher than in adults (27). Steady-state concentrations for the same mg/kg dose were correspondingly lower in children than in adults. Consistent with the higher clearance, the calculated half-life of TPM in children is approximately 15 hours without enzyme induction and 7.5 hours with enzyme induction. In young children (younger than 4 years old), clearance rates were the same or slightly higher than in older children (28). In elderly patients (65 to 85 years of age), clearance decreases only to the extent that renal function itself is reduced by age; age alone does not alter clearance in adults (29).
TPM clearance is reduced by 40% to 50% in patients with moderate (creatinine clearance, 30 to 69 mL per minute) or severe (creatinine clearance, <30 mL per minute) renal impairment compared with subjects with normal renal function (creatinine clearance, >70 mL per minute) (26). One half of the usual TPM dose is recommended in patients with moderate or severe renal impairment. Modest decreases in TPM clearance have been reported in individuals with moderate to severe hepatic impairment when compared with age- and sex-matched healthy controls; mean clearance was decreased 26% (31.8 versus 23.5 mL per minute) and half-life increased 36% (25 versus 34 hours), with parallel increases in plasma concentrations (26).
THERAPEUTIC DRUG MONITORING
Steady-state plasma concentrations of TPM are generally linear, with dose-proportional increases in plasma concentration (26). Mean plasma concentrations achieved during maintenance in randomized, controlled trials of TPM monotherapy were: 50 mg per day, 1.6 and 1.9 μg/mL; 97 mg per day, 3.8 μg/mL; 189 mg per day, 6.4 μg/mL; 313 mg per day, 11.7 μg/mL; 367 mg per day, 12.4 μg/mL (30). Studies of TPM as monotherapy have provided the opportunity to examine the relationship between TPM blood levels and clinical response. In a study comparing 50 and 500 mg per day TPM as monotherapy, plasma concentrations >9.91 μg/mL were associated with better seizure control compared with plasma concentrations of 1.77 to 9.91 μg/mL and ≤1.76 μg/mL (31). However, because of the intraindividual variations in blood levels associated with seizure control and side effects, a traditional “therapeutic range” cannot be identified. As expected, plasma concentrations are higher when TPM is administered as monotherapy (6.4 to 12.4 μg/mL with ˜200 to 400 mg per day) versus its use as add-on to enzyme-inducing AEDs (1.4 to 5.3 μg/mL with ˜200 to 400 mg per day). Despite the substantially higher plasma concentration with monotherapy, the incidence of central nervous system (CNS)-related adverse events, particularly cognitive effects, was substantially lower with TPM monotherapy than with adjunctive therapy. This finding underscores the contribution of pharmacodynamic interactions to the occurrence of adverse events during TPM polytherapy and the limited benefit of therapeutic drug monitoring in TPM-treated patients.
The relationship between TPM dose and plasma level was examined in children in whom TPM was titrated to clinical response or side effects (32). Among 21 children aged 6 to 12 years, TPM plasma levels were predictably related to dose (1:1 ratio). With monotherapy, a mean dose of 9.7 mg/kg per day (range, 5.5 to 16.5 mg/kg per day) resulted in a mean plasma level of 9.8 μg/mL (range, 3.4 to 16.6 μg/mL). For 20 younger children (younger than 6 years of age), however, higher monotherapy doses were needed (mean, 22.5 mg/kg per day; range, 11 to 35 mg/kg per day) to achieve seizure control; mean plasma level was 14.8 μg/mL (range, 6.1 to 23.7 μg/mL). When TPM was administered with an enzyme-inducing drug, the TPM dosage in younger children (mean, 14.2 mg/kg per day) was double that in older children (7.0 mg/kg per day) (32).
DRUG INTERACTIONS
Predominantly renal elimination and low protein binding minimize the potential for drug interactions.
Pharmacokinetic interactions between TPM and other AEDs are limited primarily to the effects of enzyme-inducing drugs on TPM. TPM plasma levels are approximately 50% lower when TPM is given with an enzyme-inducing AED (33, 34, 35) compared to TPM use alone or in combination with nonenzyme-inducing drugs (33, 34, 35, 36). The addition of TPM does not significantly affect plasma concentrations of carbamazepine (33), valproate (36), phenobarbital/primidone (34), or lamotrigine (37). However, phenytoin plasma levels may be increased as much as 25% in some patients, particularly those in whom phenytoin metabolism may be at or near saturation (35). Studies of TPM in models designed to predict drug interactions related to the cytochrome P450 (CYP450) enzyme system have shown inhibition of only the CYP2C19 isozyme, which may account for the potential interaction with phenytoin (38). Although pharmacokinetic interactions between TPM and other AEDs are limited, the lower incidence of adverse effects with TPM monotherapy (31,39,40) suggests that pharmacodynamic interactions may affect tolerability when TPM is added to existing therapy.
Pharmacokinetic interactions between TPM and other AEDs are limited primarily to the effects of enzyme-inducing drugs on TPM. TPM plasma levels are approximately 50% lower when TPM is given with an enzyme-inducing AED (33, 34, 35) compared to TPM use alone or in combination with nonenzyme-inducing drugs (33, 34, 35, 36). The addition of TPM does not significantly affect plasma concentrations of carbamazepine (33), valproate (36), phenobarbital/primidone (34), or lamotrigine (37). However, phenytoin plasma levels may be increased as much as 25% in some patients, particularly those in whom phenytoin metabolism may be at or near saturation (35). Studies of TPM in models designed to predict drug interactions related to the cytochrome P450 (CYP450) enzyme system have shown inhibition of only the CYP2C19 isozyme, which may account for the potential interaction with phenytoin (38). Although pharmacokinetic interactions between TPM and other AEDs are limited, the lower incidence of adverse effects with TPM monotherapy (31,39,40) suggests that pharmacodynamic interactions may affect tolerability when TPM is added to existing therapy.
Interaction studies evaluating the effect of TPM on combination oral contraceptives showed that TPM has no effect on the progestin (norethindrone, 1.0 mg) component (41,42). At doses of ≤200 mg per day, TPM has no significant effect on estrogen (ethinyl estradiol, 35 μg) concentrations (41,42). At higher doses (400 and 800 mg per day), TPM was associated with 21% and 30% reductions, respectively, in ethinyl estradiol concentrations, suggesting a modest induction of estrogen clearance (42). The level of induction is substantially less than that associated with potent enzyme-inducing agents such as carbamazepine (42% reduction in estrogen concentration) (41). The dose-related effect of TPM on estrogen clearance is consistent with the concentration-dependent induction of CYP450 CYP3A4 activity measured in vitro (43). TPM induced CYP3A4 enzymes only at concentrations >50 μM, a concentration that is unlikely to be achieved with dosages up to 400 mg per day; enzyme induction was still less than that associated with known inducers (phenobarbital and rifampicin) used in this study.
EFFICACY
Adjunctive Therapy
Partial-Onset Seizures
The effectiveness of TPM as adjunctive therapy across a wide range of doses (200 to 1,000 mg per day) in adults with refractory partial-onset seizures has been well documented in randomized, double-blind, placebo-controlled trials (46, 47, 48, 49, 50, 51, 52, 53, 54). Similarity of trial design and patient populations allowed pooled analysis of data from six of these trials (46, 47, 48, 49, 50, 51). Among 743 adults (median baseline frequency, 12 seizures per month), median seizure reduction was 44% with TPM treatment versus 2% with placebo (p ≤0.001); 43% of TPM-related patients (placebo, 12%; p ≤0.001) achieved at least 50% seizure reduction (55). During 11 to 19 weeks of double-blind treatment, 5% of patients in the TPM group were seizure free, while no patients in the placebo group were seizure free (p ≤0.001) (55). Although dosages as high as 1,000 mg per day were evaluated, the most clinically useful adjunctive therapy dosages appear to be 200 to 400 mg per day. In a 12-week, double-blind trial to further evaluate the lower end of the presumed dosing range (54), 200 mg per day TPM was added to carbamazepine. Median seizure reduction in TPM-treated patients (N = 168) was 44% (versus 20% with placebo, N = 91; p <0.001); 45% of TPM-treated patients (placebo, 24%; p = 0.001) achieved at least 50% seizure reduction. After 2 weeks, median seizure reduction in patients receiving TPM 100 mg per day (N = 84) was 60% (placebo, 17%; p <0.001), which suggests that 100 mg per day may be a target dose at which seizure control should be initially evaluated.
The initial overestimation of TPM dosage needs is evident from prospective, in-practice studies in which adults with refractory partial-onset seizures achieved good seizure control with 264 mg per day (48% of patients had 50% or more seizure reduction rate; 9% were seizure-free) (56) and 323 mg per day (68% of patients had a 50% or more seizure reduction rate) (57). When titrating to response, patients with fewer baseline seizures (less than 4 per month) required lower TPM dosages (303 mg per day) than those with higher baseline seizure frequency (341 mg per day in patients with 4 or more seizures per month) (57). In a prospective study, 17% of refractory patients had at least 50% seizure reduction and 8% were seizure-free with TPM dosages of 100 or less mg per day (58).
In treatment-resistant epilepsy patients treated at a tertiary epilepsy center, estimated long-term retention rates among 393 TPM-treated patients were 52% after 1 year, 42% at 2 years, 30% at 3 years, and 28% at 5 years (59,60). Although these rates were higher than those with another new-generation agent (lamotrigine), the low retention rate at 5 years reflects the limitations of medical therapy in patients with refractory epilepsy.
TPM was evaluated as adjunctive therapy in 86 children (2 to 16 years of age) with refractory partial-onset seizures (61). With a mean daily dose of 6 mg/kg (target dose, 5 to 9 mg/kg per day), median seizure reduction was 33% (placebo, 11%; p = 0.03). More TPM-treated children had at least 50% reduction in seizures (39% versus 20% with placebo; p = 0.08); 5% of children receiving TPM had no seizures, while no placebo-treated children were seizure free.
All 83 children completing the double-blind phase entered the long-term, open-label extension in which the dosages of TPM and concomitant AEDs could be adjusted according to clinical response (62). Mean treatment duration
was 15 months, with some children being treated as long as 2.5 years; the mean TPM dosage was 9 mg/kg per day (range, 4 to 22 mg/kg per day). Among children treated for at least 6 months, 64% had at least a 50% reduction in seizures; 14% were seizure free for a minimum of 6 months. During open-label in-practice studies in children with refractory partial-onset seizures (63, 64, 65, 66), 4% to 20% of TPM-treated children were seizure-free during treatment periods as long as 33 months.
was 15 months, with some children being treated as long as 2.5 years; the mean TPM dosage was 9 mg/kg per day (range, 4 to 22 mg/kg per day). Among children treated for at least 6 months, 64% had at least a 50% reduction in seizures; 14% were seizure free for a minimum of 6 months. During open-label in-practice studies in children with refractory partial-onset seizures (63, 64, 65, 66), 4% to 20% of TPM-treated children were seizure-free during treatment periods as long as 33 months.
Lennox-Gastaut Syndrome
TPM was evaluated as adjunctive therapy in 98 patients with Lennox-Gastaut syndrome confirmed by an electroencephalographic (EEG) pattern of slow spike-and-wave, multiple seizure types, including drop attacks, and a history of atypical absence episodes (67). At a maximum dose of 6 mg/kg per day, median reduction for drop attacks was 15% compared with a 5% increase with placebo; 28% of TPM-treated patients were responders (placebo, 14%). A combined measure of drop attacks and tonic-clonic seizures showed a 26% reduction with TPM and a 5% increase with placebo (p = 0.015); respective responder rates were 33% and 8% (p = 0.002). These outcomes compared favorably with those reported for lamotrigine in this population (68). The placebo-adjusted responder rate for drop attacks was 14% for TPM and 15% for lamotrigine; respective rates for major motor seizures were 25% and 17% (67,68).
During the long-term, open-label extension in which the dosages of TPM and concomitant AEDs could be adjusted according to clinical response (69), 55% of the 82 children treated with TPM for more than 6 months had at least a 50% reduction in drop attacks during the last 6 months of treatment; 15% experienced no drop attacks. Two patients were free of all seizures. The mean duration of TPM treatment was 18 months, with treatment periods as long as 3.4 years. The mean TPM dosage was 10 mg/kg per day (range, 1 to 29 mg/kg per day). Among patients treated as long as 8 years, 21% to 40% of patients had at least 50% seizure reduction, with major motor seizures being the most responsive (70,71).
Generalized Tonic-Clonic Seizures of Nonfocal Origin
Two double-blind, placebo-controlled trials (72,73) evaluated TPM in the treatment of generalized, nonfocal tonic-clonic seizures (i.e., primary generalized tonic-clonic seizures). Inclusion criteria specified tonic-clonic seizures with or without other generalized seizure types and electroencephalographic (EEG) or CCTV/EEG patterns consistent with generalized epilepsy (generalized, symmetric, synchronous spike-wave discharges and normal background activity); patients with Lennox-Gastaut syndrome or partial-onset seizures were excluded. In the two trials, more than 70% of patients had primary generalized tonic-clonic seizures plus at least one other type of generalized seizure (i.e., absence, myoclonic, or tonic).
TPM was initiated as adjunctive therapy in adults and children (at least 4 years of age) with refractory generalized tonic-clonic seizures despite treatment with one or two AEDs. The target dose was 5 to 9 mg/kg per day and the maximum daily dose was 400 mg. In one trial (72), baseline seizure frequency in the TPM-treated group (N = 39) was five generalized tonic-clonic seizures per month (placebo, 4.5 generalized tonic-clonic seizures per month; N = 41). Median seizure reduction was 57% (placebo, 9%; p <0.02) for tonic-clonic seizures and 42% (placebo, 1%; p = 0.003) for all generalized seizures. Among TPM-treated patients, generalized tonic-clonic seizures and all generalized seizures were reduced at least 50% in 56% and 46%, respectively (respective placebo values: 20%, p = 0.001; 17%, p = 0.003). No generalized tonic-clonic seizures occurred during the 20-week study in 13% of TPM-treated patients (placebo, 5%); 5% had no generalized seizures of any type (placebo, 0% of patients).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree