Traumatic Brain Injury
Michael J. Makley
key points
Traumatic brain injury (TBI) is the leading cause of death and disability in young adults.
The two primary mechanisms of injury in acceleration/deceleration brain trauma are focal cortical contusion and widespread neuronal damage called diffuse axonal injury (DAI).
The duration of amnesia and confusion termed posttraumatic amnesia (PTA) is the best predictor of outcome following TBI.
Disrupted sleep cycles play a major role in the cognitive and behavioral deficits that are the hallmarks of emergence from a moderate-to-severe TBI.
Although patients with TBI are at an increased risk for seizures, use of prophylactic anticonvulsants should not continue beyond one week after injury in most cases.
EPIDEMIOLOGY AND OVERVIEW
The wide spectrum of possible presentations in traumatic brain injury (TBI) offers several challenges to the clinician. Often the clinician is asked not only to manage the patient medically but also to prognosticate regarding outcome and return to former activities such as school, work, or driving. Managing each of these patients is complicated additionally by various factors that will affect outcome and recovery, such as the extent and severity of injury, age, genetic factors, premorbid learning disability, or substance abuse history. This chapter will discuss this wide spectrum of patient presentations with TBI. Topics covered will range from the patient in coma to guidelines for dealing with sports-related concussion. This overview will provide the reader with a sense of the breadth of the disease process; an understanding of some basic terminology and pathophysiology; and finally, some fundamental concepts of managing these patients at various levels of disease severity.
The aftermath of brain injury has a huge impact not only on individuals and families but also on society. It has been estimated that 1.5 to 2 million people suffer a TBI each year, with nearly a million of these being treated in an emergency room. In relative terms this is greater than
the yearly incidence of stroke, spinal cord injury, and multiple sclerosis combined. The incidence of severe TBI is 14 per 100,000 people, with nearly 60% of these people dying from their injury. The incidence for moderate and mild TBI is 15 and 131 per 100,000, respectively.
the yearly incidence of stroke, spinal cord injury, and multiple sclerosis combined. The incidence of severe TBI is 14 per 100,000 people, with nearly 60% of these people dying from their injury. The incidence for moderate and mild TBI is 15 and 131 per 100,000, respectively.
There is a bimodal distribution in regard to age and TBI. The first peak, in the second and third decade of life, is predominantly related to motor-vehicle accidents (MVA); the second peak, in the sixth decade of life, is predominantly related to falls. In the younger age groups it is a male disease (2:1), whereas in the older age groups it becomes more evenly distributed between genders. TBI is the leading cause of death and disability in young adults, affecting this population in their peak income-producing and reproductive part of their lives. The economic impact in the United States has been estimated for both direct and indirect costs of lost wages and productivity at $39 billion per year. Despite this incidence and cost to society there are very few well-controlled clinical trials to guide the clinician in the management of these patients after acute resuscitation. Often treatment is based on small trials, case reports, and anecdote. The need for the development of effective treatment is made all the more compelling with the return of soldiers from Afghanistan and Iraq. It is estimated that 10% to 20% of soldiers involved in these two wars have sustained a head injury. The enormity of the cost of long-term care and management of these veterans is only beginning to be realized.
▪ SPECIAL CLINICAL POINT: Traumatic brain injury in the younger population is primarily young males having car and motorcycle accidents while in the older population brain injury is generally related to falls and is more evenly distributed between males and females.
PATHOPHYSIOLOGY AND OPERATIONAL TERMS
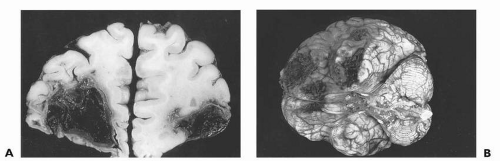
To understand TBI at any injury severity level it is important to understand some fundamental pathophysiology. The two main mechanisms of injury considered to be at work in acceleration/deceleration injury are related to impact injury or focal cortical contusion and diffuse axonal injury (DAI). In Figure 16.1, an impact injury, the point of impact is primarily the bilateral frontal and temporal poles, which correspond to the brain overlying the inside of the skull’s orbitofrontal ridge and each wing of the sphenoid bone, which cradles the temporal lobes. These areas of damage ultimately will lead to long-term behavioral sequelae of TBI as manifested by executive dysfunction, specifically deficits in behavior modulation, attention, and memory encoding and retrieval. Other focal injuries to motor, language, and visual cortex will lead to more obvious neurologic syndromes such as spastic hemiparesis, aphasia, and hemianopsia.
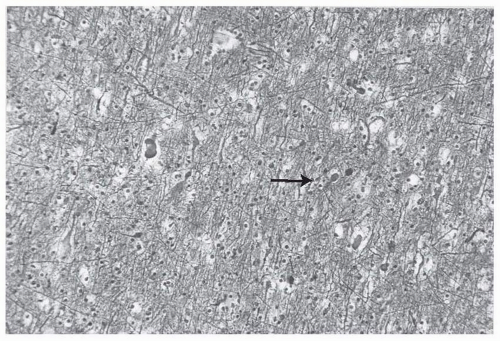
The pathologic hallmark of DAI on a cellular level is the presence of axonal retraction balls (Fig. 16.2). Originally it was thought that
these occurred with shearing forces to the axon at the moment of impact and that these “balls” were just the effect of the axon rolling up like a window shade that was wound too tightly. Postmortem studies, however, have shown that these retraction balls are not evident for 12 to 24 hours postinjury. It is now thought that impact causes a perturbation in axoplasmic transfer that, over several hours, leads to axonal swelling and finally disconnection and Wallerian degeneration. This disconnection leads to widespread deafferentation, which is thought to be one of the most significant factors in terms of long-term morbidity and recovery. Although DAI is a pathologic, postmortem diagnosis, patients who survive TBI are described as having had axonal injury when there is a prolonged loss of consciousness with a normal computed tomography (CT) scan or neuroimaging findings that show diffuse petechial intraparenchymal hemorrhage (Fig. 16.3).
these occurred with shearing forces to the axon at the moment of impact and that these “balls” were just the effect of the axon rolling up like a window shade that was wound too tightly. Postmortem studies, however, have shown that these retraction balls are not evident for 12 to 24 hours postinjury. It is now thought that impact causes a perturbation in axoplasmic transfer that, over several hours, leads to axonal swelling and finally disconnection and Wallerian degeneration. This disconnection leads to widespread deafferentation, which is thought to be one of the most significant factors in terms of long-term morbidity and recovery. Although DAI is a pathologic, postmortem diagnosis, patients who survive TBI are described as having had axonal injury when there is a prolonged loss of consciousness with a normal computed tomography (CT) scan or neuroimaging findings that show diffuse petechial intraparenchymal hemorrhage (Fig. 16.3).
▪ SPECIAL CLINICAL POINT: The primary mechanism of brain injury in acceleration/deceleration trauma is focal cortical contusion and diffuse axonal injury (DAI).
Other factors that contribute to injury severity include extra-axial and intraparenchymal hemorrhage, anoxia, and impact depolarization with widespread release of excitatory neurotransmitters and generation of free radicals. For every level of injury this secondary cascade of events also will have an impact on outcome and recovery. Anoxic injury in addition to TBI is associated with the worst outcome, and the prognosis for a good recovery is poor. Finally, in considering outcome, it is important to keep in mind that the extent of each person’s recovery from TBI may be determined by an individual’s genetic makeup. Some research suggests that patients carrying a mutant allele of apolipoprotein E have increased beta amyloid deposition in the brain and a significantly longer recovery following TBI.
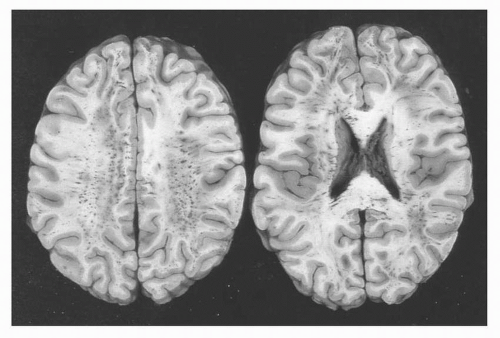 FIGURE 16.3 Diffuse white matter petechial hemorrhage seen in diffuse axonal injury (DAI) on gross section |
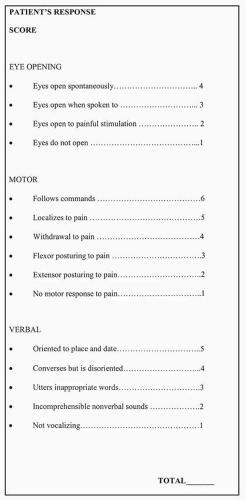
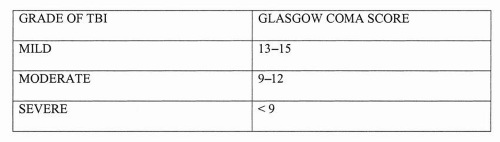
Before discussion of patient management, it is helpful to define certain terms commonly used by specialists who treat brain-injured patients. Because of the current lack of any specific functional or structural imaging test that can reliably define the extent of injury, the field has relied primarily on descriptor scales to describe brain injury severity. Two of the most commonly used scales in TBI are the Glasgow Coma Scale (GCS) and the Rancho Los Amigos Scale (RLAS) (Figs. 16.4 and 16.5). The GCS is widely used in trauma centers to describe the level of awareness in the patient with TBI. This scoring system occurs over three domains of motor, verbal response, and eye opening. The lower scores (3-8) are associated with coma or the minimally responsive patient, and the higher scores (13-15) describe a person who may be a little confused but is oriented and following commands. The GCS also has been used to grade severity of TBI (Fig. 16.6). Although commonly used to grade severity, a number of recent studies have shown that initial GCS is a poor predictor of long-term functional outcome.
The RLAS is used primarily in the postacute or rehabilitation setting, in which the bottom of the scale, I and II, is associated with a vegetative state and the top of the scale at VIII is associated with only mild cognitive deficits. This scale is a narrative description of emergence stage by stage from DAI where the duration of each stage on the scale is directly proportional to the severity of injury. In the least severe injury, such as concussion, passage through the first stages may be fairly rapid. In the more severe cases the passage up these stages can be months to years, and for some patients their progress can stall at any point on the RLAS.
Traumatic brain injury is unique among acquired brain disease because of the predominance of behavioral and memory deficits over motor and sensory deficits. Because of this, it is important to understand the concept of posttraumatic amnesia (PTA), a unique memory
disorder of TBI. PTA is a compound amnestic syndrome in which a person has lost both previously acquired memory (retrograde amnesia) as well as the ability to lay down new memory (anterograde amnesia). PTA is more than a memory disorder. It is a constellation of behavioral symptoms, which in addition to amnesia includes poor safety awareness and insight into deficits, decreased attention, as well as impulse dyscontrol. In many ways this state could be considered a delirium. Patients with ongoing PTA are very difficult to discharge into the community because of the need for 24-hour supervision. Because of this, many patients will be discharged to nursing homes or specialized behavioral units until this resolves.
disorder of TBI. PTA is a compound amnestic syndrome in which a person has lost both previously acquired memory (retrograde amnesia) as well as the ability to lay down new memory (anterograde amnesia). PTA is more than a memory disorder. It is a constellation of behavioral symptoms, which in addition to amnesia includes poor safety awareness and insight into deficits, decreased attention, as well as impulse dyscontrol. In many ways this state could be considered a delirium. Patients with ongoing PTA are very difficult to discharge into the community because of the need for 24-hour supervision. Because of this, many patients will be discharged to nursing homes or specialized behavioral units until this resolves.
Obviously the inability to lay down memory has implications for any rehabilitation-training program, but the duration of PTA also has been used to describe severity of TBI as well as predict outcome in terms of disability. One neuropsychologic screening test to determine the duration of PTA is called the Galveston Orientation and Amnesia Test (GOAT), in which a score of 75 or greater is associated with resolution of PTA. Another commonly used tool to measure PTA in the rehabilitation setting is called the Orientation Log or O-LOG. The O-LOG is less cumbersome than the GOAT and can be administered by a clinician other than a neuropsychologist. This particular test scores the patient from 0 to 30 and the threshold for clearance of PTA is a score of 25. Serial testing using either the GOAT or the O-LOG provides the length of time spent in this amnestic state. Length of PTA can be estimated retrospectively by a neuropsychologist.
While the initial GCS score is a poor prognostic marker, the duration of PTA has been linked to severity of injury, long-term functional outcomes, and resource utilization. PTA longer than 24 hours is associated with severe TBI. Data from Katz and Alexander showed that for those with PTA of less than 2 weeks, 76% reached a level of good recovery, whereas 22% were moderately disabled and 2% were severely disabled at 1 year. For survivors with 8 to 12 weeks of PTA, only 12% had a good recovery at 1 year, whereas 75% suffered moderate disability and 13% were severely disabled. No one with PTA longer than 12 weeks had what was described as a good recovery.
Information about long-range needs for patients following head injury is often identified by loved ones and families of brain-injured individuals as essential information they expect from the clinician. However, the traditionally quoted percentage breakdown between good recovery and disability, as discussed above, can often be confusing for families. Kothari and colleagues recently completed a review of the literature on TBI outcomes in order to develop evidence based “threshold values” for prognostication following head injury based on duration of both PTA and coma. These values can be helpful to the clinician in discussions with families regarding long-term outcomes. According to this review, severe disability, or being functionally dependant for most care, is unlikely with a period of PTA less than the threshold value of 2 weeks. Conversely duration of PTA greater than 3 months is unlikely to be associated with a return to previous level of functioning and independence. With regards
to the comatose patient, Kothari’s review supports the observation that the longer the duration of coma the worse the outcome. In terms of coma threshold values Kothari suggests that for coma less than 2 weeks severe disability is unlikely while a good recovery is improbable for patients in coma longer than 3 months.
to the comatose patient, Kothari’s review supports the observation that the longer the duration of coma the worse the outcome. In terms of coma threshold values Kothari suggests that for coma less than 2 weeks severe disability is unlikely while a good recovery is improbable for patients in coma longer than 3 months.
▪ SPECIAL CLINICAL POINT: Families of people with head injury identify prognostic information on long-term outcomes as the most important information they need from the clinician after the acute period. Threshold values based on duration of PTA and coma can help present this information in an understandable way.
ACUTE HOSPITALIZATION
Most patients who experience a brief loss of consciousness and present to the emergency department with a GCS of 13 to 15 will have routine evaluations that should include a complete neurologic examination, CT scan looking for evidence of extra-axial blood, and radiographic clearance of any neck injuries. Those with a normal neurologic and radiographic examination often will be sent home after a period of observation. The acute hospitalization of moderate-to-severe TBI typically will be managed in a trauma center and will be provided by a neurosurgeon or an experienced team of trauma specialists and intensivists.
In the acute hospitalized setting the primary focus of care often will be the management of increased intracranial pressure and intracranial hemorrhage. Patients with expanding epidural hematomas will be taken to the operating room immediately. Subdural and intraparenchymal blood will be assessed for evacuation according to the grade and severity as well as the underlying neurologic examination. The blood generally may be removed when it is associated with mass effect and shift of midline structures or a deteriorating neurologic examination.
With severe impact injury the secondary cascade of widespread depolarization can lead to an overwhelming increase in intracranial pressure from intraparenchymal edema. Even without an expanding mass lesion this type of diffuse swelling can rapidly lead to herniation and death. Typically neurosurgeons monitor this with an intracranial pressure monitor that is passed through the skull. Patients may be given hypertonic saline or mannitol and may be hyperventilated to bring their PCO2 down to 25 mmHg. Pentobarbital coma is often the next step when these measures fail to bring down the pressure. Even with all of these interventions, patients with severe trauma can continue to have unrelenting elevated pressures. The most drastic measure to reverse this process is craniectomy and duraplasty. This surgery removes the cranium and places a duraplastic patch on the dura that allows the brain to expand unimpeded by the calvarium. The skull flap is frozen in the pathology lab and replaced after the swelling has subsided. Although many patients survive the acute edema, carefully controlled studies have not been done for this dramatic intervention and long-term outcome data are unknown.
Another issue that arises in the acute care setting is seizure prophylaxis. People with closed-head injury (CHI) are at a greater risk of developing seizures. Various studies have found between 2% and 12% of patients with CHI will develop posttraumatic seizures. For those with dural-penetrating injuries, the rate may be more than 50%. In the early 1990s Temkin and colleagues investigated seizure prophylaxis using a double-blind, placebo-controlled paradigm in patients presenting with TBI. Their results showed there was no benefit to phenytoin after the first 7 days in terms of preventing the development of posttraumatic seizures. However, patients with penetrating head injury, because of the higher risk of developing posttraumatic seizures, should be maintained on seizure prophylaxis for 6 months to 1 year, depending on the severity of injury.
POSTACUTE HOSPITALIZATION
As methods for acute resuscitation improve with the development of acute trauma centers there has been a dramatic increase in brain-injured patients at the lowest level of responsiveness. Data from the National Traumatic Coma Data Bank demonstrated that nearly 10% of the patients discharged from an acute trauma center are in a minimally responsive state, yet there is no consensus on their appropriate management. Often these patients are discharged to nursing homes poorly equipped and trained to manage the complex needs of these patients. Although functional goals are limited in the minimally responsive patient, there is a role for an interdisciplinary approach to the management of these low-level patients (i.e., RLAS I to II), particularly just after acute hospitalization. In one study, 60% of patients admitted to a specialized low-level head-injury program emerged and progressed to an acute rehabilitation program with age being the most significant predictor of successful emergence.
In these low-level patients the most important medical management issues revolve around complications that come from an immobile, minimally responsive patient. Such a patient needs provision of aggressive pulmonary toilet, treatment of infections, prevention of decubitus ulcers, maintenance of adequate nutrition, management of spasticity, and access to comprehensive family education. The role of the interdisciplinary therapy team within a specialized low-level brain injury program is not only for passive range of motion and sensory stimulation. Trained therapists also assess the person’s level of responsiveness to his or her environment using a systematic scoring tool such as the JFK Coma Recovery Score or other scoring system. The physician, in addition to treating medical issues and spasticity, often will introduce various neurostimulants, such as methylphenidate, amantadine, bromocriptine, or levodopa/carbidopa (Table 16.1). These agents are thought to enhance arousal and attention primarily through augmentation of the dopaminergic system. Another agent used to treat attention-deficit disorder called atomoxetine is getting wide use in the low-level brain-injured patient. This agent is thought to primarily work at the noradrenergic receptor by selectively inhibiting reuptake of norepinephrine. Exactly how these neurostimulants work in the low-level TBI patient is not clear, although they may correct a severe disturbance of sleep-wake cycle. Although there is widespread use of these agents in this patient
population, there are no large-scale, placebocontrolled trials that guide their use.
population, there are no large-scale, placebocontrolled trials that guide their use.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree