aAvoid concomitant use with the lowest dose oral contraceptive pills, and additional nonhormonal forms of contraception should be used.
PREGNANCY
Epilepsy is the most common neurologic disorder that requires continuous treatment during pregnancy, and AEDs are one of the most frequent chronic teratogen exposures. Approximately one-half million women with epilepsy are of childbearing age in the United States, and 3 to 5 births per 1000 will be to women with epilepsy (3). However, it is estimated that the total number of children in the United States exposed in utero to AEDs is substantially greater with the emergence of AED use for other illnesses including headache, chronic pain, and mood disorders.
Treatment during pregnancy is a precarious balancing act between the teratogenic risks of AEDs and maintaining maternal seizure control. However, pregnancy registries and more intensive observational studies have provided key data that allow us to lower the risk for the developing fetus to rates closer to the general population. These findings should be key considerations when counseling and treating adolescents and women with epilepsy during their reproductive years (3–5).
MAJOR CONGENITAL MALFORMATIONS
Offspring of women with epilepsy on AEDs are at an increased risk for major congenital malformations (MCMs) and minor anomalies. Minor anomalies are defined as structural deviations from the norm that do not constitute a threat to health. Minor anomalies affect 6% to 20% of infants born to women with epilepsy, approximately 2.5-fold the rate of the general population. Although not of direct health consequence, the finding of a minor anomaly should lead to enhanced vigilance about the child’s health and neurodevelopment.
Major congenital malformations (MCMs) are defined as an abnormality of an essential anatomical structure present at birth that interferes significantly with function and/or requires major intervention. The reported MCM rates in the general population vary between 1.6% and 3.2%, and women with a history of epilepsy but on no AEDs show similar MCM rates. The average MCM rates among all AED exposures vary between 3.1% and 9%, or approximately two- to threefold higher than the general population (3,6).
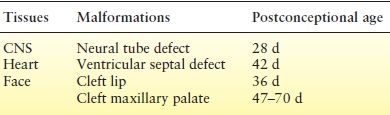
The MCMs most commonly reported following in utero AED exposure include congenital heart disease, cleft lip/palate, urogenital defects, skeletal defects, and neural tube defects. The abnormal neural tube closure usually occurs between the 3rd and 4th weeks of gestation. By the time most women realize they are pregnant, it is too late to make medication adjustments to avoid malformations (Table 47.2).
Table 47.2 Relative Timing and Developmental Pathology of Certain Major Congenital Malformations
AED Monotherapies
The information we have gained regarding specific AEDs and risk for MCMs has increased dramatically over the last two decades. Data obtained from large, prospective pregnancy registries from different parts of the world have demonstrated remarkably consistent findings for many of the AEDs. The 2009 AAN Practice Parameter updates on “Management issues for women with epilepsy—focus on pregnancy” (3) led to many important conclusions about intrauterine first-trimester exposure and risk for MCMs: (i) It is highly probable that valproic acid (VPA) exposure has higher risk of MCMs compared to CBZ and possible compared to PHT or lamotrigine (LTG). (ii) Compared to untreated women with epilepsy (WWE) it is probable that VPA as part of polytherapy and possible that VPA as monotherapy contribute to the development of MCMs. (iii) It is probable that AED polytherapy as compared to monotherapy regimens contributes to the development of MCMs. (iv) CBZ probably does not substantially increase the risk of MCMs in the offspring of WWE. (v) There is probably a relationship between the doses of VPA and of LTG and the risk of development of MCMs in the offspring of WWE. Additionally, for specific types of MCMS, findings included the following: (i) PHT possibly contributes to the risk of cleft palate, (ii) CBZ possibly contributes to the risk of posterior cleft palate, (iii) VPA probably contributes to neural tube defects and facial clefts and possibly contributes to hypospadias, and (iv) PB possibly contributes to cardiac malformations.
Since this evidence-based review of the literature, several large prospective pregnancy registries scattered across different regions of the world continue to provide valuable information. They reveal a very consistent pattern of amplified risk for the development of MCM in pregnancies exposed to VPA. The registries have also provided updated information on additional AEDs that further refines our ability to lower the teratogenicity risk in women with epilepsy.
The UK Epilepsy and Pregnancy Register reported on findings with TPM use in 178 live births (7). Although the confidence intervals were wide, this preliminary information noted an MCM rate of 4.8% for monotherapy use and even higher for use of TPM as polytherapy. They also noted a particularly higher rate of oral clefts, approximately 11 times their background rate, and a high rate of hypospadias. The risk of oral clefts with TPM has been replicated in other studies (8).
Using data from the National Birth Defects Prevention Study, Werler et al. (9) reported that increased risks were observed for VPA and neural tube defects (OR, 9.7; 95% CI, 3.4 to 27.5), oral clefts (OR, 4.4; 95% CI, 1.6 to 12.2), heart defects (OR, 2.0; 95% CI, 0.78 to 5.3), and hypospadias (OR, 2.4; 95% CI, 0.62 to 9.0). Increased risks were observed for CBZ and neural tube defects (OR, 5.0; 95% CI, 1.9 to 12.7). Similarly, the European Surveillance of Congenital Anomalies (EUROCAT) antiepileptic-study database, which is derived from population-based congenital anomaly registries, also reported significantly increased risks for VPA monotherapy and spina bifida, atrial septal defect, cleft palate, hypospadias, polydactyly, and craniosynostosis (10). Spina bifida was the only specific MCM associated with CBZ monotherapy compared with no AEDs (OR, 2.6; 95% CI, 1.2 to 5.3), but the risk was smaller than for VPA (11,12).
The North American AED Pregnancy Registry released findings comparing the risk of MCMs among infants exposed to different AED monotherapies during the first trimester, as well as to an unexposed reference group (13). The LTG monotherapy group was chosen as the exposed reference group for the other AEDs because of a low MCM rate and tight confidence intervals (2.0% [95% CI, 1.4 to 2.8]). Table 47.3 in this article can serve as a particularly instructive tool during the preconceptional counseling phase of women with epilepsy, with detailed information on many of the AEDs with sample size and calculation of confidence intervals for the risk numbers presented. Additional analysis included the risk of MCM by average daily VPA dose during the first trimester and confirms prior reports of a dose-related risk for VPA and MCM. However, the upper limit of the confidence intervals for the lowest VPA daily dosage group (<500 mg) reached over 7%, and this is an uncommon dose range to maintain seizure control.
Table 47.3 Risk of major congenital malformations identified among infants who had been exposed to a specific AED monotherapy regimen during the first trimester and relative risk of MCMs compared to both unexposed and to lamotrigine groups: North America Pregnancy Registry 1997–2011
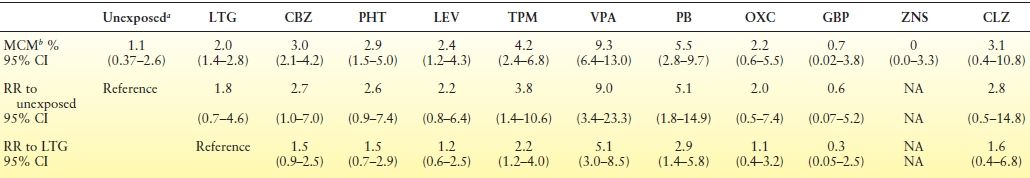
aThe unexposed internal comparison group were pregnant women not taking an AED, who were recruited from among the friends and family members of the enrolled women taking an AED.
bDiagnosed during pregnancy or before 12 wks after birth. Confirmed by review of medical records.
AED, antiepileptic drug; MCM, major congenital malformations; RR, relative risk; CI, confidence interval; LTG, lamotrigine; CBZ, carbamazepine; PHT, phenytoin; LEV, levetiracetam; TPM, topiramate; VPA, valproate; PB, phenobarbital; OXC, oxcarbazepine; GBP, gabapentin; ZNS, zonisamide; CLZ, clonazepam.
Adapted from Hernández-Díaz S, Smith CR, Shen A, et al.; North American AED Pregnancy Registry; North American AED Pregnancy Registry. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78(21):1692–1699.
Hernandez-Diaz et al. (13) also examined the frequency of specific MCMs for each AED and reported that VPA was associated with an increased risk of hypospadias, neural tube defects, and cardiovascular malformations; PB was associated with an increased risk of cardiovascular malformations; and the risk of oral clefts was higher among infants exposed to PB, VPA, and TPM, consistent with previous reports.
The UK and Ireland Epilepsy and Pregnancy Registers combined results for first-trimester exposure to levetiracetam (LEV) with outcome data for 304 monotherapy pregnancies and 367 polytherapy pregnancies (14). The MCM rate in the LEV monotherapy group was 0.70% (95% confidence interval [CI], 0.19% to 2.51%) and in the polytherapy group was 5.56% (95% CI, 3.54% to 8.56%); the MCM rate in the polytherapy group was lower when LEV was given with LTG (1.77%; 95% CI, 0.49% to 6.22%) than when given with VPA (6.90%; 95% CI, 1.91% to 21.96%) or CBZ (9.38%; 95% CI, 4.37% to 18.98%).
A recent report from the European and International Registry of Antiepileptic Drugs and Pregnancy (EURAP) confirmed that in addition to the type of AED, dose of the AED at conception also affects rates of MCMs (15). MCM rates in pregnancies exposed to CBZ, LTG, VPA, and PB were analyzed by dose at time of conception (not throughout the first trimester or entire pregnancy). The lowest rates of MCMs occurred with LTG < 300 mg/day (2.0%; 95% CI, 1.19 to 3.24), and this group was used as the comparator group. Risks of MCMs were higher with VPA and PB at all doses and with CBZ at >400 mg/day. Additionally, an increase in MCM rates was observed with increasing doses for all of the four AEDs (Fig. 47.1).
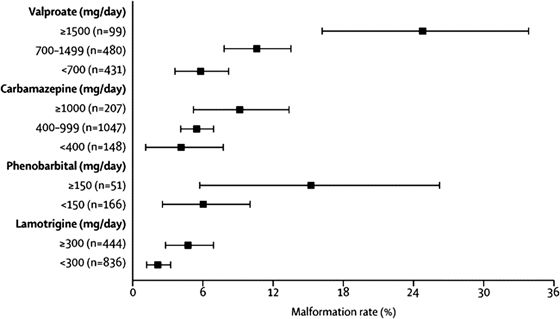
Figure 47.1. Rates of major congenital malformations at 1 year after birth in relation to exposure to AED monotherapy according to data from the International Registry of Antiepileptic Drugs and Pregnancy. Bars represent 95% confidence interval. (From Tomson T, Battino D, Bonizzoni E, et al.; for the EURAP study group. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10(7):609–617.)
Recent data support the concern that the amount of fetal exposure to an AED is important, as well as the type of AED. Therefore, reduction of the dose prior to conception while maintaining seizure control can further reduce the risk of structural teratogenicity. Determining the women’s individual target concentration preconception can be a valuable tool for therapeutic drug monitoring during pregnancy (see below).
AED Polytherapy
The rates of MCMs have been reported as higher across several studies for women on AED polytherapy compared to AED monotherapy regimens (3). These results led to the recommendation that AED monotherapy is preferred to polytherapy during pregnancy and should be achieved during the preconception planning phase (3). However, Holmes et al. (16) reported that data from the North American AED Pregnancy Registry suggest that not all AED polytherapy combinations are alike. Concentrating on LTG or CBZ as polytherapy, both AEDs had relatively modest rates for MCMs if the polytherapy combination was with any AED other than VPA. The MCM rates were 9.1% for LTG plus VPA (OR, 5.0; 95% CI, 1.5 to 14.0 compared with LTG monotherapy) but only 2.9% for LTG with any other AED (1.5; 0.7 to 3.0); likewise, the risks were 15.4% for CBZ plus VPA (OR, 6.2; 95% CI, 2.0 to 16.5 compared with CBZ monotherapy) and 2.5% for CBZ plus any other AED (0.8; 0.3 to 1.9).
NEURODEVELOPMENTAL OUTCOMES
Studies investigating cognitive outcome in children of women with epilepsy report an increased risk of mental deficiency (3,17). Verbal scores on neuropsychometric measures may be selectively more involved. A variety of factors contribute to the cognitive problems of children of mothers with epilepsy, but AEDs appear to play a major role. Factors other than specific AED use that have been associated with cognitive impairment include seizures, a high number of minor anomalies, major malformations, decreased maternal education, impaired maternal–child relations, and maternal focal seizure disorder (17). It is possible that these risk factors are not only additive but synergistic.
The 2009 AAN Practice Parameter Updates reported the following conclusions about in utero exposure (throughout the entire pregnancy) and risk for poor cognitive outcomes (3): (i) cognition is probably not reduced in children of untreated WWE, (ii) CBZ probably does not increase poor cognitive outcomes compared to unexposed controls, (iii) monotherapy exposure to VPA probably reduces cognitive outcomes, (iv) monotherapy exposure to PHT or PB possibly reduces cognitive outcomes, and (v) AED polytherapy exposure probably reduces cognitive outcomes as compared to AED monotherapy.
Since the 2009 AAN Practice Parameter update, several notable reports have contributed to our understanding of the various contributors to adverse neurodevelopmental outcomes and the pattern seen. The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study was a prospective, observational, multicenter study in the United States and United Kingdom and assessed the neurodevelopmental effects of in utero exposure to four monotherapy groups (CBZ, VPA, PHT, and LTG) (18). The primary outcome was intelligence quotient (IQ) at age 6 years, adjusted for maternal IQ, AED type, AED-standardized dose, gestational age at birth, and use of periconceptional folate. Primary analysis included 305 mothers and 311 children, with 224 children completing the 6-year follow-up. Multivariate analysis demonstrated that the VPA-exposed children had lower age-6 IQ compared to CBZ, LTG, or PHT, and they did poorly on several specific measures. High doses of VPA were negatively correlated with IQ, verbal ability, nonverbal ability, memory, and executive function, while the other AEDs did not have a dose effect. Interestingly, mean IQs were higher in the children of mothers who took periconceptional folic acid (Fig. 47.2). This key evidence of a beneficial effect of supplemental folic acid taken prior to and early in pregnancy in women with epilepsy on AEDs supports the recommendation that all women of childbearing age should be encouraged to take supplemental folic acid given the high unplanned pregnancy rate.
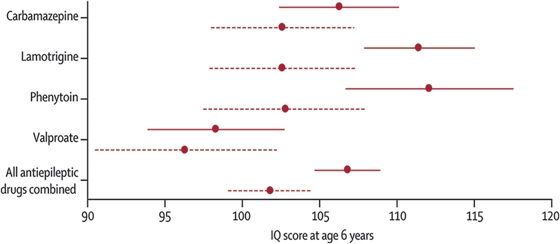
Figure 47.2. Child IQ at 6 years of age, by exposure to maternal AED use and periconceptional folate. Mean (95% confidence intervals) are shown for folate (solid lines) and no folate (dashed lines). (IQ, intelligence quotient.) (From Meador KJ, Baker GA, Browning N, et al.; for the NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244–252.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








