Vascular Diseases in Forensic Neuropathology
Juan C. Troncoso
Cerebrovascular diseases and lesions are common in forensic neuropathology. As described earlier, subarachnoid and parenchymal hemorrhages are frequently encountered in traumatic brain injury (Chapter 7) and hemorrhagic infarcts as complications of cerebral herniations (Chapter 8). In this chapter, we will focus first on naturally occurring cerebrovascular diseases (i.e., hemorrhages and infarcts) and then on vascular lesions related to trauma.
HEMORRHAGES OF THE BRAIN
The most frequent nontraumatic hemorrhages of the brain examined at forensic institutions are subarachnoid and parenchymal hemorrhages presenting as sudden death.
Subarachnoid Hemorrhage
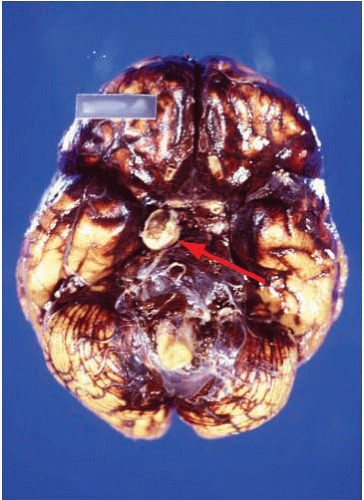
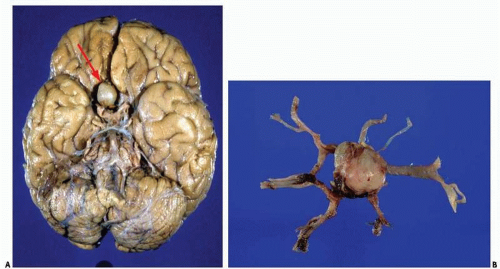
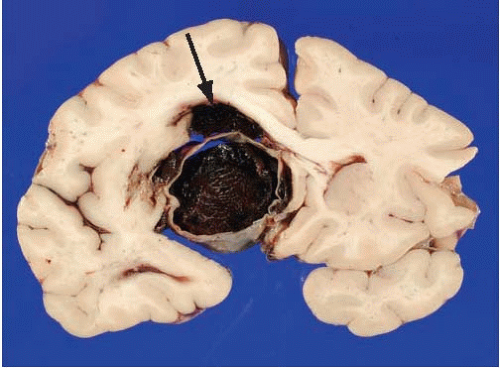
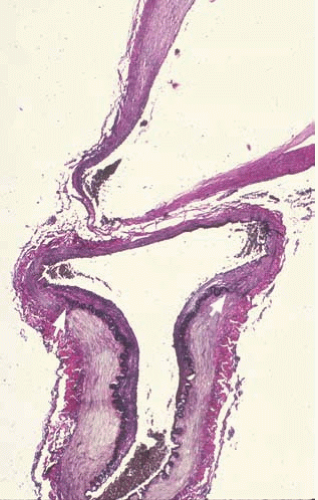
Subarachnoid hemorrhages (SAHs) can be primary or traumatic (as discussed in Chapter 7). In a forensic setting, primary SAH is usually the consequence of a ruptured intracranial berry (saccular) aneurysm and often presents as sudden and unexpected death in adults1 (Fig. 10.1). Berry aneurysms are focal dilations of arterial vessels that occur usually at branching points and at times are found incidentally at autopsy (Fig. 10.2). Usually, ruptured berry aneurysms bleed directly into the subarachnoid space, but occasionally, the jet of blood is directed into the brain parenchyma or ventricular system and may or may not extend into the subarachnoid space (Fig. 10.3). The current concept is that the aneurysms develop at the site of a congenital or acquired gap of the media or elastic lamina, compounded by hemodynamic stress (Fig. 10.4).2 Consistent with this tenet, the incidence of berry aneurysms increases with age, with a 1% prevalence in the overall population, 2% in middle-aged individuals, and 5% in older subjects. Although aneurysmal SAH in children is exceptional, it has been described.3, 4 We have seen cases of ruptured aneurysms in individuals as young as 8 years of age (see Fig. 14.8), but the majority of the cases are in subjects older than 25 years. Most berry aneurysms are single, although multiple aneurysms have been reported in up to 20% of cases, 5 and most are sporadic, but they are familial in 5% of cases. Familial aneurysms are frequently multiple. Genetic factors play an important role in the pathogenesis of intracranial aneurysms. Recent and ongoing studies are focusing on mutations and polymorphisms of genes coding for proteins of the vessel wall (i.e., extracellular matrix, elastin, and collagen).6 Intracranial aneurysms are associated with various diseases, such as polycystic kidney disease, coarctation of the aorta, Marfan syndrome, fibromuscular dysplasia, and moyamoya disease.1
In a 15-year survey of 133 cases of ruptured berry aneurysms examined at the office of the Chief Medical Examiner in Baltimore between year 1989 and year 2003, 71 subjects (53%) were women between the ages of 26 and 76 years (mean age, 45 years), and 62 subjects (47%) were men between the ages of 18 and 67 years (mean, age 44.5 years).7 Most often, death is sudden, and it may occur unwitnessed or during sleep. Occasionally, a history is present of severe headache immediately before the ictus, and, at times, we have examined cases with histories of premonitory headaches for 1 or 2 days. In our series, 59% of the subjects were found unresponsive, 14% had a witnessed collapse, and 7% presented with headache, neck, or back pain. In terms of relation of the subarachnoid hemorrhage to activity, some occur during exercise, sexual intercourse, or cocaine abuse; however, a large number of cases are unrelated to exercise and many occur during sleep.
Forensic Studies
Experience with berry aneurysms and acute SAHs at a forensic institution will be different from that in a general hospital or specialized medical center. A study from the Mayo Clinic identified all patients with aneurysmal SAH in Rochester, Minnesota, between 1960 and 1989. There were 80 women and 33 men, with a mean age of 55 years. Among the 113 patients, 13 (12%) died suddenly without reaching the hospital. The frequency of posterior circulation aneurysms in this group was 38%, compared with 14% for those admitted to the hospital. Another important feature of the sudden death group was intraventricular hemorrhage in 92% and pulmonary edema in 92%.8
In a review of 219 cases of aneurysmal subarachnoid hemorrhage autopsied at the Dallas County Medical Examiner’s Office between 1977 and 1997, 56% were women and 44% were men and age range was 31 to 70 years, with the highest frequency (29%)
between ages 41 and 50 years. The most common location for the ruptured aneurysm was the middle cerebral artery distribution (39%), and multiple aneurysms were present in 9.1% of the cases.
between ages 41 and 50 years. The most common location for the ruptured aneurysm was the middle cerebral artery distribution (39%), and multiple aneurysms were present in 9.1% of the cases.
In our experience, the SAH caused by a ruptured aneurysm extends into the ventricular system in 63% of the cases and has intraparenchymal extension in 20% of the cases.
Observations at Autopsy
After careful external examination of the head and neck to exclude trauma, examination of the brain reveals diffuse fresh SAH, usually most abundant at the base rather than over the convexity of the brain, but frequently extending along the Sylvian fissures (Fig. 10.1). Because examination of blood vessels is critical in these cases, it is of paramount importance to remove the brain with great care and to avoid tearing the arteries at the base of the brain by applying unnecessarily strong traction. Once the brain is removed, as much blood as possible should be removed from the base of the brain by using a gentle jet of water or hydrogen peroxide. Frequently, with this maneuver, the circle of Willis becomes visible and the ruptured aneurysm can be identified immediately. The brain is then placed in formalin for fixation. This is recommended even if an aneurysm was already identified to exclude additional aneurysms and neuropathologic changes.
Observations at Brain Cutting
The SAH is usually abundant at the base of the brain, in the Sylvian fissures, and, of variable magnitude, over the cerebral convexity. The distribution of the hemorrhage is important because at times it is most abundant close to the point of bleeding. For example, heavy hemorrhage at the base of the brainstem and interpeduncular fossa is common in ruptured aneurysms of the basilar artery, whereas a thick clot in the chiasmatic cistern and orbitofrontal regions usually indicates a lesion of the anterior cerebral or anterior communicating arteries. After standard external examination, description, and documentation of the subarachnoid hemorrhage, the blood vessels are carefully examined in situ by using a fine forceps, cotton swabs, and good lighting. The search for an aneurysm can be challenging, and is frequently time consuming. A magnifying glass can be of great help when dealing with difficult cases and small aneurysms.
In our experience, the most common locations for aneurysms are the anterior cerebral-anterior communicating arteries (38%), the internal carotid-posterior communicating arteries (27%), the middle cerebral artery or its proximal branches (16%), the basilar artery (8%), or the vertebral arteries (8%).7 Our approach is first to examine the vertebrobasilar system, followed by the internal carotid-posterior communicating arteries, middle cerebral arteries, and, finally, the anterior cerebral-anterior communicating arteries. Frequently, the examination requires the careful opening of the Sylvian fissures to inspect the middle cerebral arteries and the separation of the gyri recti to visualize the anterior cerebral-anterior communicating arterial complex. Injection of a dye solution through a cannula in the internal carotid or vertebral arteries can also be of help in identifying a ruptured aneurysm.
Once the aneurysm is identified, the vessels are then removed, and we search for additional aneurysms. If the inspection of the blood vessels in situ does not reveal an obvious lesion, then we proceed to careful dissection of the circle of Willis and other arteries at the base of the brain. At times, dissection of the vessels has helped us to find the ruptured aneurysm not obvious on in situ inspection, but it has the disadvantage that the handling and pulling of vessels may create artifactual tears of the vessel walls that could be mistaken for the base of a ruptured aneurysm.
After external and vascular examination, the brain is cut coronally, and there is usually a variable amount of fresh intraventricular hemorrhage (63% of cases in our experience), which tends to be more abundant in the fourth than in the third or lateral ventricles. This is blood that has entered retrogradely from the subarachnoid space into the ventricular system. Sometimes the jet of blood emerging from the ruptured aneurysm may dissect through the parenchyma of the brain and reach the ventricular system. This occurrence is most common with hemorrhages originating from the anterior communicating artery (Fig. 10.3). Rarely, the hemorrhage may be confined to the parenchyma of the brain and ventricular system, sparing the subarachnoid space. Occasionally we have noted ruptured aneurysms in the coronal sections that could not be identified on external examination. Although we pay particular attention to vascular lesions in cases of SAH, a thorough inspection for other types of lesions should not be neglected.
In a few instances, subjects who die from an SAH have had previous surgery for the ruptured aneurysm or for another one, in cases of multiple aneurysms. In these circumstances, the presence, number, and location of surgical clips or intravascular devices (coils) should be identified.9
Other vascular malformations, such as arteriovenous malformations and cavernous angiomas, may also be the source of SAH. These lesions, however, usually have a major intraparenchymal component and are readily identifiable on examination of the brain (Fig. 10.5 and 10.6). Some of these vascular malformations may have been operated on and may have metal surgical clips or show embolization with plastic beads (Fig. 10.6).
Mechanisms of Sudden Death in Primary Subarachnoid Hemorrhage
In some instances of SAH, substantial intraventricular hemorrhage and hydrocephalus or brain edema are present that allow us to implicate increased intracranial pressure as the mechanism of death. In many cases, however, the hemorrhage may be limited to the subarachnoid space, and the brain shows no edema and no sign of herniation. In these circumstances, neurogenic pulmonary edema, cardiac arrhythmias, ormyocardial injury may offer a better explanation.8
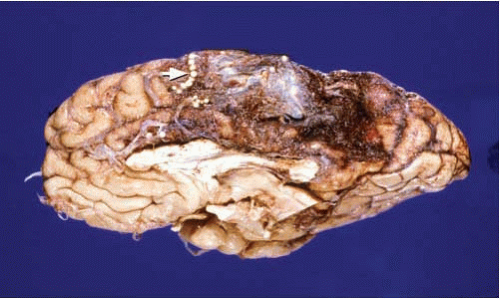
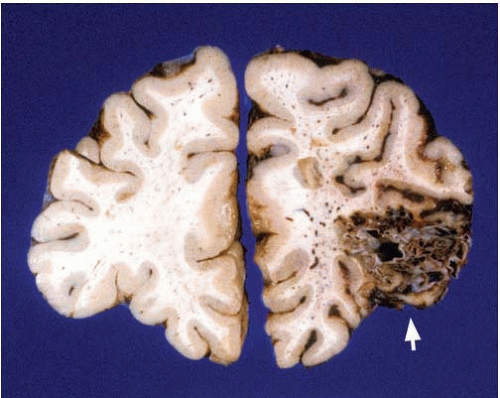 FIG. 10.5. Arteriovenous malformation of the right inferior frontal gyrus (arrow). Note the large variability on the caliber of the blood vessels within the malformation. |
Experimental SAH in spontaneously breathing cynomolgus monkeys causes reductions in cerebral blood flow and respiratory abnormalities consisting of apnea, hyperventilation, and hypoxemia.10 In rats, experimental SAH results in increased intracranial pressure, decreased cerebral perfusion, increase in arterial blood pressure, and concomitant decrease in heart rate and electrocardiographic changes. Interestingly, all of these changes can also be elicited by injection of saline into the cisterna magna, 11 suggesting that the development of these abnormalities is independent of the type of fluid injected into the subarachnoid space. Cardiac changes in human SAH are also well documented. A study based on electrocardiograms and echocardiograms in patients with acute SAH showed motion abnormalities in the left ventricular wall in 8% of patients, frequently associated with mild elevation of creatine kinase (myocardial band [CK-MB]) and electrocardiographic repolarization abnormalities.12 Another study showed depression of ST segment, abnormal T waves, sinus tachycardia, and right bundlebranch block.13 Furthermore, impaired left ventricular function after SAH contributes to pulmonary edema, as well as cardiovascular instability and cerebral ischemia.14
The development of neurogenic pulmonary edema in SAH has been well documented in experimental and clinical studies, but there is no consensus on its pathogenesis. In subjects who die suddenly from SAH, more than 90% present with acute pulmonary edema.15 Among those who survive acute SAH and are admitted to the hospital, 6% to 21% will develop pulmonary edema. Subjects with more severe SAH tend to develop pulmonary edema more frequently. The mean interval between the onset of SAH and diagnosis of pulmonary edema on chest radiograph is 2.5 hours.13 Regarding the mechanisms of neurogenic pulmonary edema, acute and fulminating pulmonary hemorrhagic edema occurs in association with the severe hypertension and bradycardia of the Cushing response after intracranial hypertension. Notably, the lung pathology can be prevented by spinal transection and sympathoadrenergic blocking agents, but is not affected by decerebration, adrenalectomy, vagotomy, or atropine. The central sympathetic activation resulting from increased intracranial pressure causes two major hemodynamic changes: (1) an increase in vascular resistance, with a reduction in the vascular capacity of the systemic and pulmonary circulation, and (2) acute left ventricular failure, resulting in volume and pressure loading in the pulmonary circulation.16 A recent study of acute subarachnoid hemorrhage in rats suggests that the development of neurogenic pulmonary edema is characterized by increased capillary permeability to proteins and is independent of the degree of intracranial pressure increase or the type of fluid injected into the subarachnoid space. This same study suggests that an inappropriate Cushing response to increased intracranial pressure may be fatal to experimental animals.15
Primary Parenchymal Hemorrhages of the Brain
This is a common finding in forensic neuropathology. It generally presents as sudden death, and it occurs frequently during sleep; at other times, the unconscious individual is taken in agonal state to the emergency room. Although we see it mainly in individuals older than 40 years, it may occur in younger individuals. The groups at higher risk of parenchymal hemorrhage are cocaine addicts and individuals with histories of or autopsy findings (i.e., left ventricular hypertrophy, granular kidneys) consistent with longstanding arterial hypertension.
External Examination of the Brain
The most common pathologic changes are SAH, mass effect, and herniation. Herniations are frequently noted under the falx cerebri (cingulate herniation) and/or transtentorial (uncal herniation). When the hemorrhage is cerebellar, tonsillar herniation is common. Subarachnoid hemorrhage, if present, is usually located at the base of the pons and cerebellum, indicating its exit through the foramina of Luschka and Magendie. Nevertheless, occasionally the cerebral or pontine hemorrhage dissects through the parenchyma and opens into the subarachnoid space. In the cerebrum, the rupture is usually through the lateral surface of the temporal or frontal lobes, and it is important to differentiate it from a traumatic laceration.
Internal Examination of the Brain
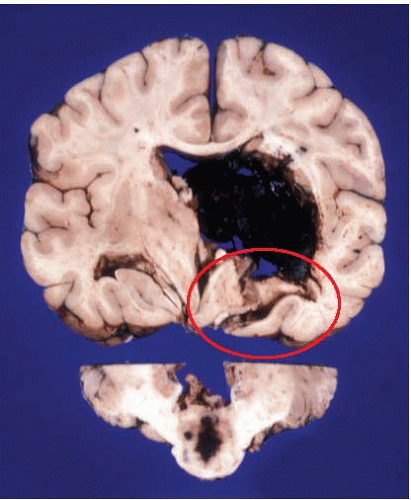
Hemorrhages are usually seen in the basal ganglia (Figs. 10.7 and 10.8) and deep white matter, thalamus (Fig. 10.9), pons (Fig. 10.10), and cerebellum (Figs. 10.11 and 10.12). Subcortical and lobar hemorrhages are rare (Fig. 10.13). Multiple hemorrhages are extremely uncommon, except for leukemias (Fig. 10.14),
coagulopathies (Fig. 10.15), tumors (Fig. 10.16), and trauma (Fig. 10.17). The hematoma hardens with formalin fixation and is difficult to cut, presenting a potential hazard to the inexperienced pathologist. Small hemorrhages remain confined to the parenchyma, but larger ones frequently dissect through and inundate the ventricular system. A minority of hemorrhages break into the subarachnoid space. In addition to the previously described herniations, it is important to examine for central herniation, characterized by elongation of the third ventricle and hypothalamus.
coagulopathies (Fig. 10.15), tumors (Fig. 10.16), and trauma (Fig. 10.17). The hematoma hardens with formalin fixation and is difficult to cut, presenting a potential hazard to the inexperienced pathologist. Small hemorrhages remain confined to the parenchyma, but larger ones frequently dissect through and inundate the ventricular system. A minority of hemorrhages break into the subarachnoid space. In addition to the previously described herniations, it is important to examine for central herniation, characterized by elongation of the third ventricle and hypothalamus.
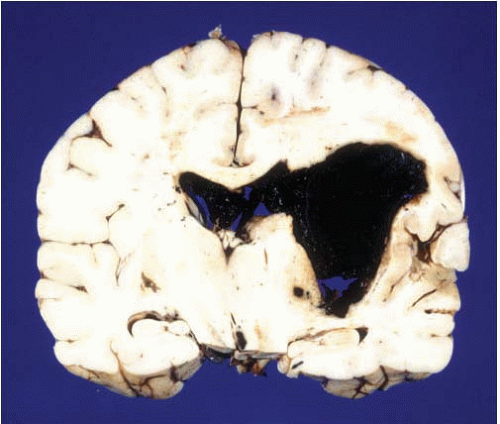 FIG. 10.7. This is a primary hemorrhage of the right basal ganglia, most likely arising from the putamen and dissecting into the ventricular system. The patient had a history of chronic hypertension. |
Although in the great majority of cases of parenchymal hemorrhages the cause can be attributed to chronic systemic hypertension, other causes should also be considered, such as vascular malformations (Fig. 10.12), berry aneurysms that rupture into the parenchyma of the brain (Fig. 10.3), angiopathies (i.e., amyloid) (Fig. 10.18), leukemias (Fig. 10.14), blood dyscrasias (e.g., thrombotic thrombocytopenic purpura or disseminated intravascular coagulation) (Fig 10.15), anticoagulation treatment, tumors (Fig. 10.16), and trauma (Fig. 10.17). This last category may be difficult to distinguish from a primary hemorrhage, because it is not uncommon that
patients who fall as a result of a cerebral hemorrhage or any other reason may suffer some degree of head trauma with scalp contusions. The best differential feature for a traumatic hemorrhage is the presence of cortical contusions and/or lacerations close to the parenchymal hematoma. Microscopic examination of tissues surrounding the parenchymal hematoma is helpful in establishing the cause of the hemorrhage. Hematoxylin-eosin stain is usually enough, but a special technique to detect amyloid angiopathy (Congo Red, thioflavin, or immunostain for β-amyloidmay be of additional help (Fig. 10.18).
patients who fall as a result of a cerebral hemorrhage or any other reason may suffer some degree of head trauma with scalp contusions. The best differential feature for a traumatic hemorrhage is the presence of cortical contusions and/or lacerations close to the parenchymal hematoma. Microscopic examination of tissues surrounding the parenchymal hematoma is helpful in establishing the cause of the hemorrhage. Hematoxylin-eosin stain is usually enough, but a special technique to detect amyloid angiopathy (Congo Red, thioflavin, or immunostain for β-amyloidmay be of additional help (Fig. 10.18).
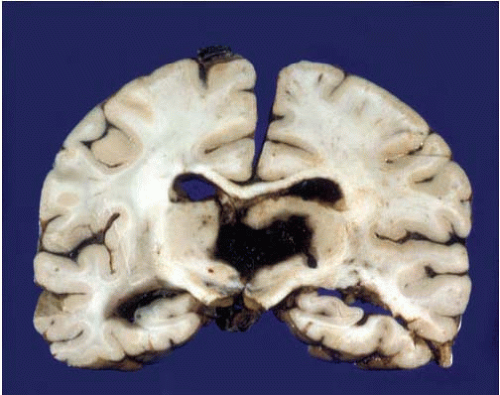
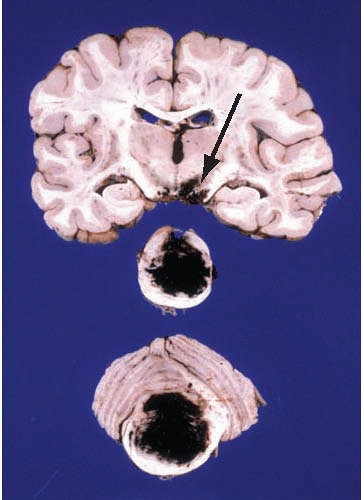 FIG. 10.10. Primary hemorrhage of the pons involving the basis and tegmentum of the pons and the fourth ventricle. The hemorrhage has also dissected rostrally into the midbrain (arrow). Note the difference of this primary hemorrhage with the Duret hemorrhage shown in Figure 10.8. |
 FIG. 10.11. Primary hemorrhage of the right cerebellar hemisphere. The hemorrhage originated in the white matter and deep nuclei. This individual had a history of chronic hypertension. |
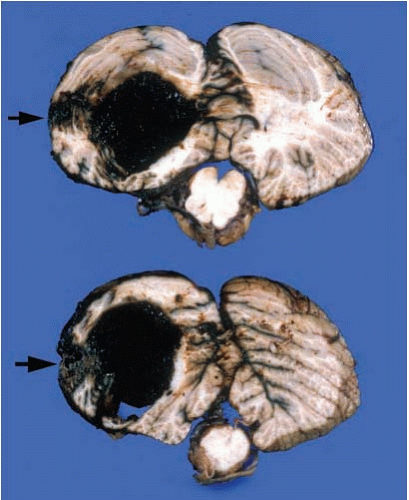 FIG. 10.12. Hemorrhage of the left cerebellar hemisphere originating from a vascular malformation of the folia (arrows).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|