23 Vascular Pathology of the Spine
I. Key Points
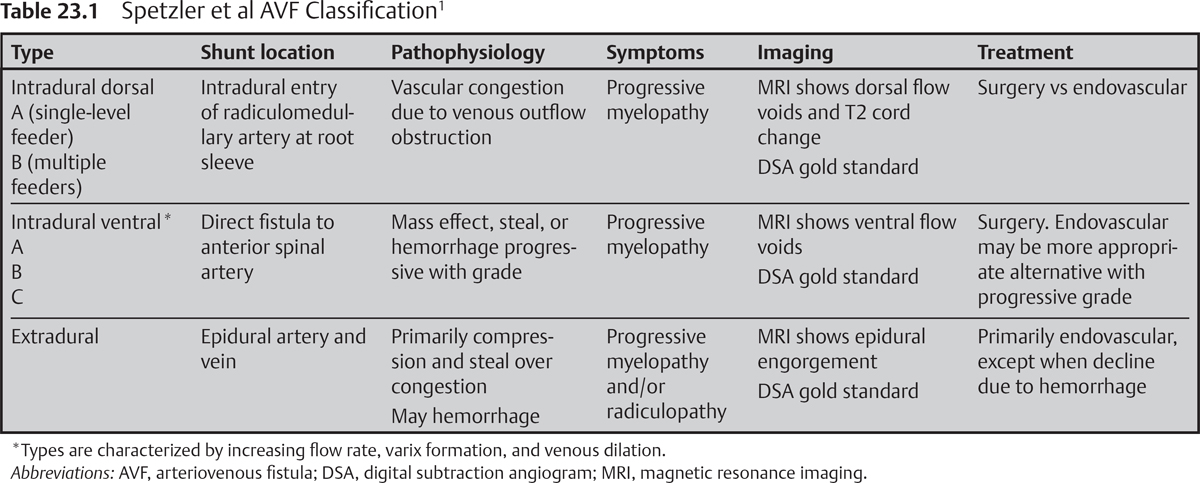
– The Spetzler et al nomenclature for spinal vascular lesions classifies arteriovenous malformations (AVMs) and arteriovenous fistulas (AVFs) according to anatomic location. Lesion classification does not rigidly dictate optimal treatment strategy but may provide a useful framework to guide decision making.1
– Spinal angiography is the gold standard and is warranted for all arteriovenous lesions.
– Thorough understanding of spinal angiographic anatomy, surgical vascular anatomy, and segmental variability is essential for decision making.
– A high index of suspicion should be maintained in the presence of spinal vascular lesions. Protean clinical findings, imaging appearance, and low incidence often result in diagnostic delay.
II. Cavernous Malformations
– Background
• Cavernous malformations are benign vascular neoplasms that may occur in sporadic or familial forms. Spinal cavernomas favor thoracic over cervical locations, with lumbar next in frequency.
• Intramedullary is the most common location, although intramedullary exophytic, intradural extramedullary, and extradural locations have been reported.
• Peak incidence of symptomatic hemorrhage is reported in the fourth decade.
– Signs, symptoms and physical examination
• Acute: due to large hemorrhage, may result in long-tract dysfunction or radicular symptoms (including pain) depending on location; acute meningeal signs are rare.
• Progressive decline or stepwise deterioration: due to repeated hemorrhages and/or hemosiderin toxicity; improvement between events is usually incomplete.
• Estimated bleed rates have been reported at 1.4 to 4.5% per patient-year. The rate of subsequent hemorrhage may approach 66% per patient-year.
– Workup
• Magnetic resonance imaging (MRI) with gradient-recalled echo (GRE) sequences
• Detailed family history, MRI of the brain, and possible familial screening should be considered. As many as 50% of patients may harbor intracranial cavernomas as well.2
– Neuroimaging
• MRI is the modality of choice. Cavernomas are angiographically occult.
• MRI appearance: T1 and T2 “popcorn” heterogeneity reflects vascular sinusoids containing blood products of different ages. Surrounding T1 and T2 hypointensity reflects the rim of hemosiderin-stained parenchyma. Hypointense “blooming” notable on GRE sequences, with absence of flow voids.3
– Treatment
• Gross total excision is the treatment goal and is protective against future hemorrhage.
• Conservative management is a consideration for small asymptomatic lesions, especially deep-seated lesions that fail to reach the pial surface on axial images.2,4
– Surgical pearls (see also Chapter 64)
• Appropriate zones of entry from a posterior or posterolateral approach include midline myelotomy, dorsal lateral sulcus, or laterally between the dentate ligaments.
• Whenever possible, sharp dissection is preferred. Piecemeal excision is common.
• Care is necessary to preserve hemosiderin-stained parenchyma surrounding the cavernoma. The developmental venous anomaly should be preserved whenever possible.2,4
III. Arteriovenous Lesion
– Background
• Intradural-dorsal AVF
 Considered an acquired lesion, attributed to venous outflow dysfunction
Considered an acquired lesion, attributed to venous outflow dysfunction
 Low-flow fistula involves radiculomedullary artery as it pierces the dural root sleeve, resulting in arterialized coronal venous plexus. Type B lesions recruit feeders from adjacent levels, but a single fistulous point is always present.
Low-flow fistula involves radiculomedullary artery as it pierces the dural root sleeve, resulting in arterialized coronal venous plexus. Type B lesions recruit feeders from adjacent levels, but a single fistulous point is always present.
 Accounts for 60 to 80% of spinal vascular lesions. Typically affects males more than females, ages 40 to 60, and has predilection for the thoracolumbar spine.
Accounts for 60 to 80% of spinal vascular lesions. Typically affects males more than females, ages 40 to 60, and has predilection for the thoracolumbar spine.
• Intradural ventral
 High-flow anastomosis between anterior spinal artery (ASA) and ventral venous plexus. Varix formation, flow rate, complexity, and multiplicity of feeding pedicles increase with types A to C.
High-flow anastomosis between anterior spinal artery (ASA) and ventral venous plexus. Varix formation, flow rate, complexity, and multiplicity of feeding pedicles increase with types A to C.
 Occurs in younger patients (20 to 60 years) and favors the thoracolumbar spine
Occurs in younger patients (20 to 60 years) and favors the thoracolumbar spine
• Extradural
 High-flow direct anastomosis between epidural artery and vein, may receive multisegmental arterial contributions1
High-flow direct anastomosis between epidural artery and vein, may receive multisegmental arterial contributions1
 Sporadic de novo formation, congenital, syndromic associations (e.g., neurofibrosis (NF)-1), and traumatic etiologies have all been reported (Table 23.1).
Sporadic de novo formation, congenital, syndromic associations (e.g., neurofibrosis (NF)-1), and traumatic etiologies have all been reported (Table 23.1).
• Extradural-intradural AVM
 High-flow AVM, irrespective of tissue boundaries. Commonly involves multiple or entire spinal segments. May interdigitate with functional cord tissue. Commonly fed by ASA and posterior spinal artery (PSA). Rare.
High-flow AVM, irrespective of tissue boundaries. Commonly involves multiple or entire spinal segments. May interdigitate with functional cord tissue. Commonly fed by ASA and posterior spinal artery (PSA). Rare.
• Intramedullary AVM
 High-flow AVM with diffuse and compact forms. High-risk features, including varix formation and associated aneurysms, are common.
High-flow AVM with diffuse and compact forms. High-risk features, including varix formation and associated aneurysms, are common.
 Typically symptomatic early in life (10 to 30 years), with predilection for the cervical spine depending on report; represents 15 to 20% of all spinal vascular lesions
Typically symptomatic early in life (10 to 30 years), with predilection for the cervical spine depending on report; represents 15 to 20% of all spinal vascular lesions
• Conus AVM: high-flow, complex shunting pattern found at conus. Multiple shunts and nidi may be pial or intramedullary. Rare (Table 23.2).1
– Signs, symptoms, and physical examination
• Intradural-dorsal AVF: protean history of progressive myelopathy, dominated by gait and sphincter dysfunction. Pathophysiology relates to venous vascular congestion.
• Intradural ventral: similarly protean, progressive, and variable history dominated by myelopathic findings. Symptomatic progression due to steal, compression, and hemorrhage increases with grade and flow rate.
• Extradural: Myeloradiculopathy is common, with symptoms due to compression, steal, and hemorrhage. Venous congestion is typically rare.
• Extradural-intradural AVM: malignant natural history characterized by progressive myelopathy. Radicular pain referable to the involved segment is typical. Pathophysiology involves mass effect, steal, hemorrhage, and compression.
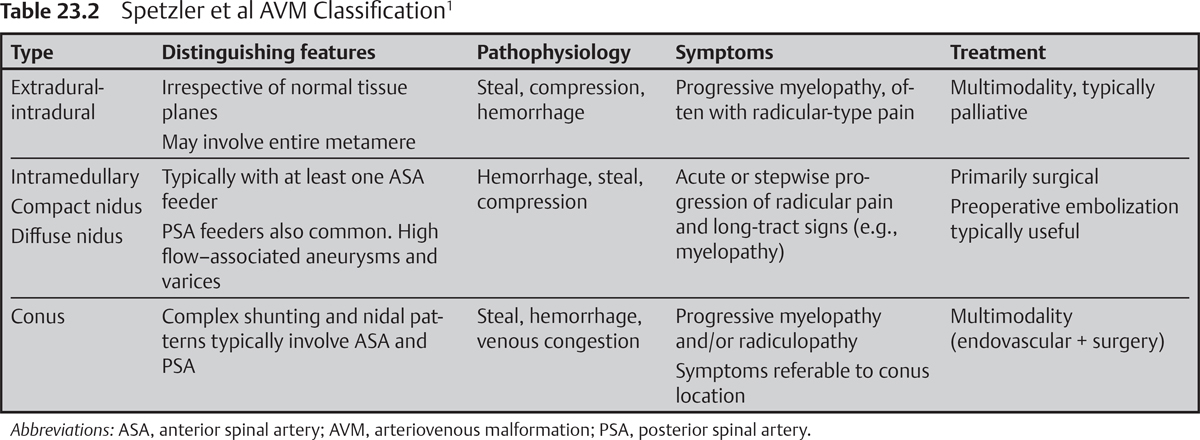
• Intramedullary AVM: malignant natural history characterized by progressive myeloradiculopathy. Stepwise deterioration or acute decline attributable to repeated hemorrhage, steal, and compression.
• Conus AVM: progressive myeloradiculopathy, symptoms referable to conus1
– Workup
• Spinal MRI/magnetic resonance angiography (MRA) has proven especially useful for intradural dorsal lesions. MRA may be sufficiently sensitive to identify the fistula type, to pinpoint the level(s) of the shunt, and to confirm treatment at follow-up.
• Spinal angiography, however, remains the gold standard imaging modality (for all spinal AVFs and AVMs) and may be directed more precisely after careful inspection of prior MRA.
– Neuroimaging
• AVMs
 MRI is most useful to delineate the size and configuration of the nidus (compact vs diffuse), to evaluate for hemorrhage, and to assess the angioarchitecture.
MRI is most useful to delineate the size and configuration of the nidus (compact vs diffuse), to evaluate for hemorrhage, and to assess the angioarchitecture.
• Intradural dorsal AVF
 MRI: In the appropriate clinical setting, extensive intramedullary T2 hyperintensity along with intradural flow voids along the dorsal pial surface is nearly pathognomonic. Enhancement may be variable.
MRI: In the appropriate clinical setting, extensive intramedullary T2 hyperintensity along with intradural flow voids along the dorsal pial surface is nearly pathognomonic. Enhancement may be variable.
 Angiography: Venous outflow is sluggish, and long venous phase may be necessary. Suspected but occult intraduraldorsal fistulas require surgical exploration.
Angiography: Venous outflow is sluggish, and long venous phase may be necessary. Suspected but occult intraduraldorsal fistulas require surgical exploration.
• Intradural ventral
 MRI: reveals variable T2 hyperintensity and/or enhancement with dilated flow voids along the ventral surface of the spinal cord. Identifies varix formation.
MRI: reveals variable T2 hyperintensity and/or enhancement with dilated flow voids along the ventral surface of the spinal cord. Identifies varix formation.
 Angiography: reveals a direct fistula between the radiculomedullary artery and ASA. The ASA is identified by the classic hairpin loop and may be displaced from midline. The ventral arterialized vein often harbors a prominent venous varix.
Angiography: reveals a direct fistula between the radiculomedullary artery and ASA. The ASA is identified by the classic hairpin loop and may be displaced from midline. The ventral arterialized vein often harbors a prominent venous varix.
• Extradural: MRI/MRA: prominent epidural flow voids and enhancement, typically with significant mass effect. Variable T2 intramedullary signal change.3
– Treatment
• Intradural-dorsal AVF: Microsurgical clip occlusion at intradural fistulous point remains the gold standard. Embolization with liquid agent now acceptable first-line treatment, but associated with greater risk of recurrence.5
• Intradural-ventral AVF: Microsurgical fistula obliteration remains treatment of choice, but embolic strategies often used as well. Vessel caliber (particularly type A and B) and proximity to ASA present challenges to endovascular management.
• Extradural AVF: Large-caliber feeding vessels favor coil embolization.
• Extradural-intradural AVM: Curative resection is atypical. Multimodal palliation strategies ameliorate symptoms due to steal, compression, and/or hemorrhage.
• Intramedullary AVM: Surgical resection, typically via a posterior or posterolateral approach, remains the gold standard and is typically preceded by attempts at embolization in appropriately selected patients.6
• Conus AVM: Multimodal strategies include aggressive embolization and resection.1
– Surgical pearls
• Indocyanine green angiography (ICG) is ideal for intraoperative confirmation of location and essential for the identification of angiographically occult lesions.
• Intramedullary AVMs are commonly fed by at least one branch of the ASA. Steal phenomena may obscure ASA involvement on preoperative angiography, but serial runs mid-resection may reveal the ASA as shunting is progressively eliminated.
• Arterial supply is circumferentially addressed first for intramedullary AVMs. Venous outflow is spared until the lesion is sufficiently devascularized.
Common Clinical Questions
1. The Spetzler et al nomenclature divides arteriovenous lesions solely on the basis of what characteristic?
2. What imaging modality is the gold standard and is mandatory for all spinal arteriovenous lesions?
3. Describe the typical imaging findings of an intradural-dorsal fistula on MRI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







