Chapter 5 Ventricles and Cerebrospinal Fluid
The cavity of the embryonic neural tube develops into a continuous, fluid-filled system of ventricles lined with ependymal cells in adults; each division of the central nervous system (CNS) contains a portion of this ventricular system. Cerebrospinal fluid (CSF) is formed within the ventricles, fills them, and emerges from apertures in the fourth ventricle to fill the subarachnoid space. CSF is responsible for suspension of the brain through its partial flotation, as discussed in Chapter 4, but it does much more than this—it is an important component of the system that regulates the composition of the fluid bathing the neurons and glial cells of the CNS and provides a route through which certain chemical messengers can be widely distributed in the nervous system.
The Brain Contains Four Ventricles
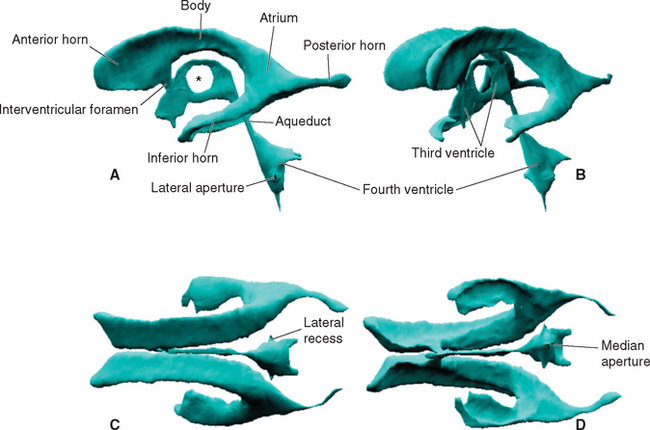
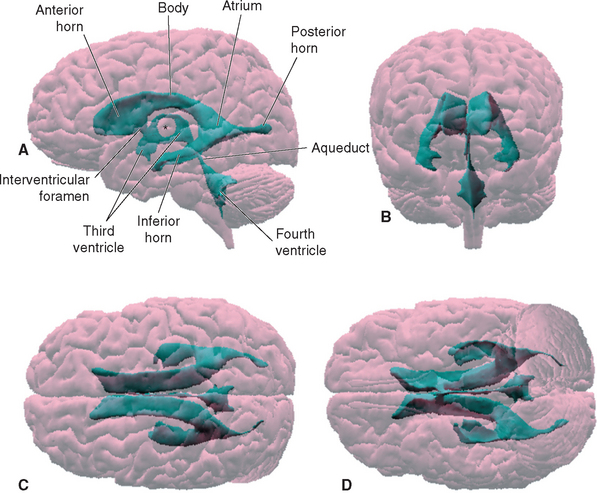
Within each cerebral hemisphere is a relatively large lateral ventricle. The paired lateral ventricles communicate with the third ventricle of the diencephalon through the interventricular foramina (foramina of Monro). The third ventricle in turn communicates with the fourth ventricle of the pons and medulla through the narrow cerebral aqueduct (aqueduct of Sylvius) of the midbrain. The fourth ventricle continues caudally as the tiny central canal of the caudal medulla and spinal cord; this canal is usually not patent over much of its extent.
A Lateral Ventricle Curves through Each Cerebral Hemisphere
Each lateral ventricle follows a long C-shaped course through all the lobes of the cerebral hemisphere in which it resides. It is customarily divided into five parts (Figs. 5-1 and 5-2): (1) an anterior (or frontal) horn in the frontal lobe, anterior to the interventricular foramen; (2) a body in the frontal and parietal lobes, extending posteriorly to the region of the splenium of the corpus callosum; (3) a posterior (or occipital) horn projecting backward into the occipital lobe; (4) an inferior (or temporal) horn curving down and forward into the temporal lobe; and (5) an atrium, or trigone, the region near the splenium where the body and the posterior and inferior horns meet. The body, atrium, and inferior horn of the ventricle represent the original C-shaped development of the lateral ventricle; the anterior and posterior horns are extensions from this basic shape.
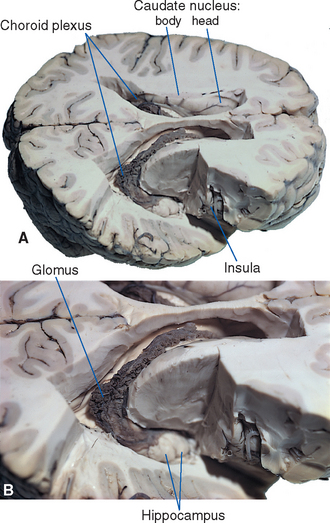
Various structures form the borders of the lateral ventricle in its course through the cerebral hemisphere; many of them can be seen easily in coronal sections (see Figs. 3-19 to 3-24 Fig. 3-19 to 3-24) or in brains dissected from above (Fig. 5-3). The similarly C-shaped caudate nucleus (see Fig. 3-18) is a constant feature in sections through the ventricle. Its enlarged head forms the lateral wall of the anterior horn (see Fig. 3-19), its somewhat smaller body forms most of the lateral wall of the body of the ventricle (see Fig. 3-21), and its attenuated tail lies in the roof of the inferior horn (see Figs. 3-22 and 5-8C). Proceeding posteriorly, as the caudate nucleus becomes smaller, the thalamus becomes larger and forms the floor of the body of the ventricle (compare Figs. 3-20 and 3-22). The corpus callosum and septum pellucidum give a good indication of the size and location of the anterior horn and body of the ventricle. The body of the corpus callosum forms the roof of these parts of the ventricle, and the genu of the corpus callosum curves down to form the anterior wall of the anterior horn. The septum pellucidum forms the medial wall of the body and anterior horn, and its termination near the splenium marks the site where the bodies of the ventricles diverge from the midline and begin to curve around into the inferior horns (compare Figs. 3-21 and 3-23).
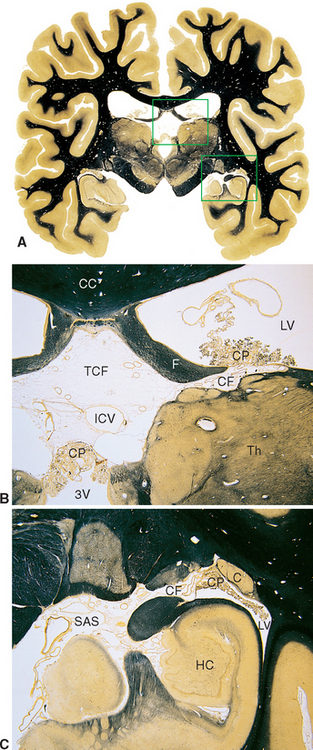
Figure 5-8 Coronal sections at different magnifications demonstrating how choroid plexus faces subarachnoid space on one side and ventricular space on the other. The areas outlined in A are enlarged in B and C. In B, choroid plexus (CP) separates the subarachnoid space of the transverse cerebral fissure (TCF) from the intraventricular spaces of the lateral ventricle (LV) and third ventricle (3V). Similarly, in C, choroid plexus (CP) separates subarachnoid space (SAS) of the ambient cistern from the intraventricular space of the inferior horn of the lateral ventricle (LV). The site of invagination of the choroid plexus in the medial wall of the lateral ventricle is the choroid fissure (CF). C, tail of the caudate nucleus; CC, corpus callosum; F, fornix; HC, hippocampus; ICV, internal cerebral vein (a major tributary of the great vein; see Chapter 6); Th, thalamus.
(A, from Nolte J, Angevine JB Jr: The human brain in photographs and diagrams, ed 3, St. Louis, 2007, Mosby.)
The posterior horn is phylogenetically the most recently developed part of the lateral ventricle and is also the most variable in size, sometimes being rudimentary. There are a number of slight asymmetries between the cerebral hemispheres of the human brain, and the left posterior horn tends to be longer than the right, particularly in right-handed individuals. The two lateral ventricles are otherwise quite symmetrical.
The hippocampus forms most of the floor and medial wall of the inferior horn (see Fig. 5-8C), which ends anteriorly at about the level of the uncus.
The Third Ventricle Is a Midline Cavity in the Diencephalon
The narrow, slit-shaped third ventricle occupies most of the midline region of the diencephalon (Figs. 5-1 and 5-2), so its entire outline can be seen in a hemisected brain (see Fig. 3-15). It often looks like a misshapen doughnut in casts or reconstructions of the ventricular system (Fig. 5-1). The hole in the doughnut corresponds to the interthalamic adhesion, which crosses the ventricle in most human brains.
An outline of the third ventricle reveals four protrusions, called recesses (Fig. 5-4), corresponding to structures that have evaginated from the diencephalon. Inferiorly the optic recess lies in front of the optic chiasm at the base of the lamina terminalis; the infundibular recess lies just behind the chiasm. Superiorly the pineal recess invades the stalk of the pineal gland, and the suprapineal recess lies just anterior to this stalk.
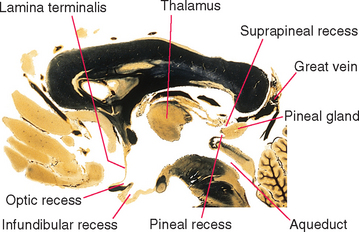
Figure 5-4 Recesses of the third ventricle, as seen in a sagittal section near the midline. The great vein (of Galen) is the principal vein draining deep cerebral structures (see Chapter 6). It empties into the straight sinus.
(Adapted from Nolte J, Angevine JB Jr: The human brain in photographs and diagrams, ed 3, St. Louis, 2007, Mosby.)
The Fourth Ventricle Communicates with Subarachnoid Cisterns
The fourth ventricle is sandwiched between the cerebellum posteriorly and the pons and rostral medulla anteriorly (Fig. 5-2). It is shaped like a tent with a doubly peaked roof, the peaks protruding into the cerebellum. The floor is relatively flat, and because it narrows rostrally into the aqueduct and caudally into the central canal, it is somewhat diamond shaped (see Fig. 11-3A). For this reason, the floor is sometimes referred to as the rhomboid fossa. At the location where the lateral point of the diamond would be expected, the entire ventricle becomes a narrow tube that proceeds anteriorly and curves around the brainstem, ending adjacent to the flocculus of the cerebellum. This tubular prolongation is the lateral recess of the fourth ventricle (Fig. 5-1). The portion of the roof of the ventricle rostral to the peak is the superior medullary velum, and the portion caudal to the peak is the inferior medullary velum. The superior medullary velum is a thin layer of white matter related to the cerebellum, whereas the inferior medullary velum is a membrane containing choroid plexus, similar to the roof of the third ventricle.
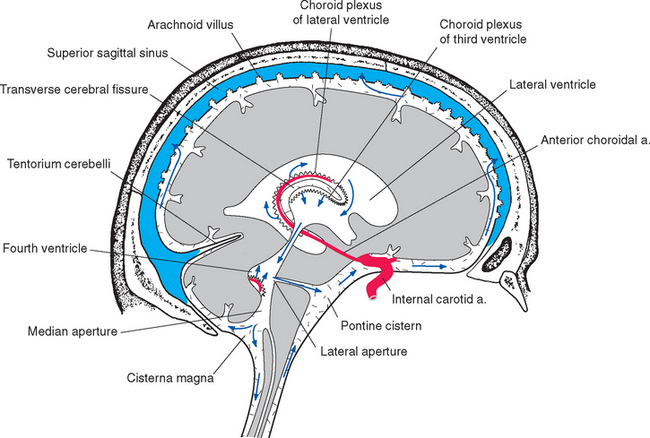
The lateral and third ventricles are nearly closed cavities, communicating only with other parts of the ventricular system. In contrast, there are three apertures in the fourth ventricle through which the ventricular system communicates freely with subarachnoid space. These are the unpaired median aperture (or foramen of Magendie) and the two lateral apertures (or foramina of Luschka) of the fourth ventricle (see Fig. 5-10). The median aperture is simply a hole in the inferior medullary velum (Fig. 5-5); it is as though the caudal end of the membrane, where it should have closed off the ventricle at its junction with the central canal, was instead lifted up and attached to the inferior surface of the cerebellar vermis. The result is a funnel-shaped opening from the subarachnoid space (the cerebellomedullary cistern, or cisterna magna) into the ventricle. The inferior medullary velum also covers the lateral recess, and at the end of each recess is another opening in the velum, the lateral aperture.
The Ventricles Contain Only a Fraction of the CSF
The ventricles are both smaller and more variable in size than one might expect. Although there is an average total of approximately 200 mL of CSF within and around the brain and spinal cord, only about 25 mL of this fluid is contained within the ventricles. The rest occupies subarachnoid space. The third and fourth ventricles together have a volume of only about 2 mL, and the volumes of the aqueduct and central canal are negligible, so the lateral ventricles contain nearly all the ventricular CSF. The total volume of 25 mL is only an average figure, and the ventricles of some apparently normal brains have been found to have total volumes of less than 10 mL or more than 30 mL (however, volumes greater than 30 mL are usually considered suspicious).
Choroid Plexus Is the Source of Most CSF
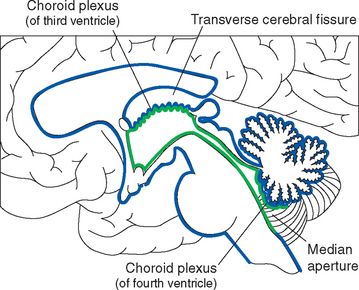
All four ventricles contain strands of highly convoluted and vascular membranous material called choroid plexus that secretes most of the CSF. The composition of choroid plexus can be appreciated by first considering, for example, the anatomy of the roof of the third ventricle (see Figs. 2-19 and 5-5). This roof is simply a layer of ependymal cells overlain by a layer of pia. As in all other locations, the pial layer also faces subarachnoid space, where the brain’s blood supply is located. At certain locations this pia-ependyma complex invaginates into the ventricle with a collection of arterioles, venules, and capillaries (Fig. 5-6). Here the ependymal layer is specialized as a cuboidal, secretory epithelium, the choroid epithelium; the whole ependyma-pia-capillary complex is the choroid plexus. There is a long, continuous band of choroid plexus reflecting the original C-shaped course of each lateral ventricle, extending from near the tip of the inferior horn, through the body of the ventricle, and reaching the interventricular foramen (Figs. 5-3 and 5-7). There is no choroid plexus in the anterior or posterior horn. The plexus is enlarged in the region of the atrium, and here it is called the glomus (Latin for “ball of thread”). Choroid plexus becomes calcified with age, and the glomus can often be seen in x-ray studies (see Fig. 5-15D). The choroid plexus of each lateral ventricle grows through the interventricular foramen, forming part of its posterior wall, and becomes one of the two narrow strands of choroid plexus in the roof of the third ventricle (Fig. 5-7). It does not continue through the aqueduct, which is lined with ependyma and completely surrounded by neural tissue.
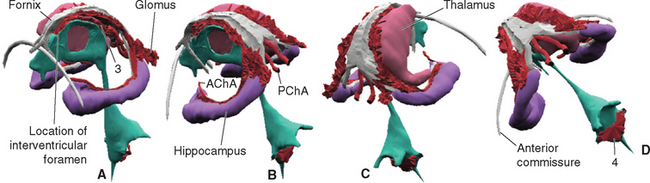
Figure 5-7 Three-dimensional reconstruction showing the location of choroid plexus, seen from the left and front (A), left and rear (B), right and rear (C), and obliquely above and behind (D). The cerebral hemispheres, lateral ventricles, and left thalamus were removed for clarity. A C-shaped strand of choroid plexus curves around with the medial wall of the inferior horn and body of each lateral ventricle, forms part of the wall of the interventricular foramen, and continues into the roof of the third ventricle (3). Separate strands of choroid plexus invaginate the roof of the fourth ventricle (4). The arterial supply of the cerebral choroid plexus (described further in Chapter 6) is provided mainly by the anterior and posterior choroidal arteries (AChA and PChA, respectively).
The choroid plexus of the fourth ventricle is formed from a similar invagination of the inferior medullary velum into the caudal half of the ventricle. It is T shaped, with the vertical part of the T consisting of two adjacent longitudinal strands of plexus. These frequently extend as far as the median aperture, where they are directly exposed to subarachnoid space (Fig. 5-5). The transverse portion of the T consists of one strand of plexus, which extends into each lateral recess. Each end reaches a lateral aperture, where a small tuft of choroid plexus generally protrudes through the aperture and is exposed directly to subarachnoid space.
Because one side of pia mater always faces subarachnoid space, choroid plexus must always be adjacent to subarachnoid space on its pial side and to intraventricular space on its choroid epithelial side. Although this may seem contrary to the plexus’s apparent location deep within each cerebral hemisphere (Fig. 5-3), it can be easily demonstrated in coronal sections (Fig. 5-8). The location of the invagination of choroid plexus into the lateral ventricle is called the choroid fissure. The choroid fissure is a C-shaped slit of subarachnoid space that accompanies the fornix system of fibers from the inferior horn to the interventricular foramen. By the same reasoning, the space above the roof of the third ventricle, which continues laterally into the choroid fissure, is also subarachnoid space (Figs. 5-5 and 5-8). This is the transverse cerebral fissure, a long finger of subarachnoid space trapped in the middle of the cerebrum by growth of the cerebral hemispheres posteriorly over the diencephalon and brainstem. The transverse cerebral fissure continues posteriorly into the superior cistern.
The Ependymal Lining of Choroid Plexus Is Specialized as a Secretory Epithelium
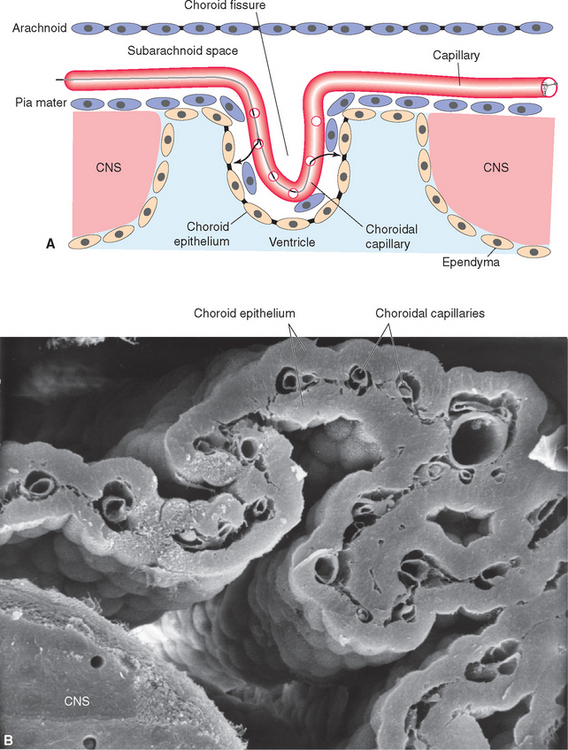
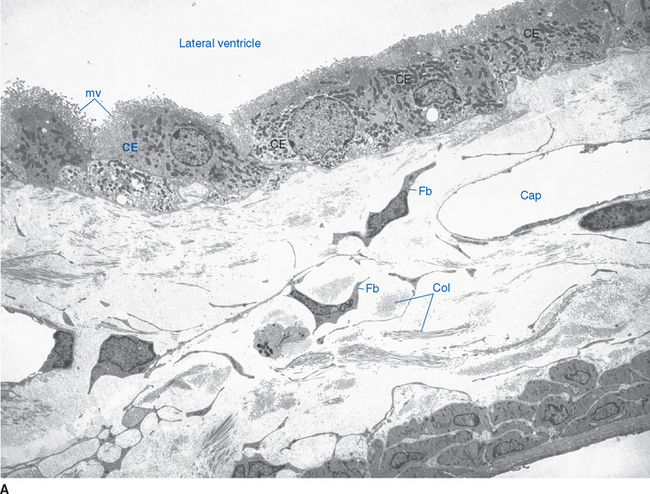
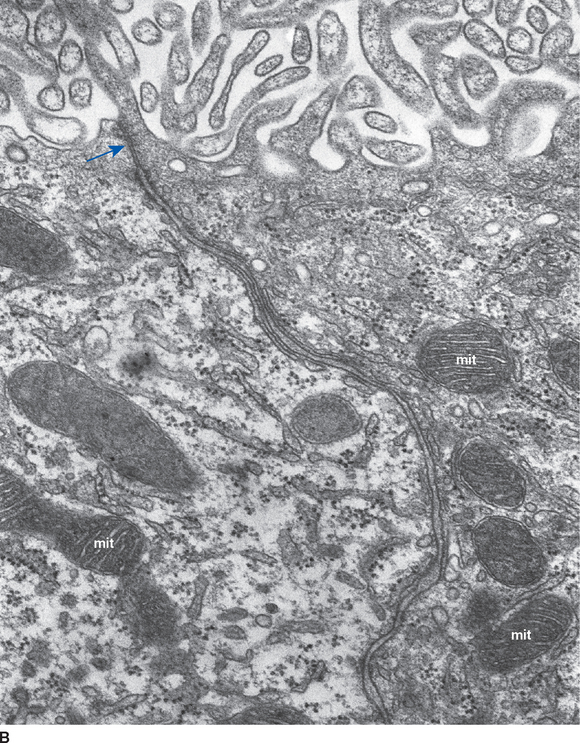
Choroid plexus is functionally a three-layered membrane between blood and CSF (Fig. 5-9). The first layer is the endothelial wall of each choroidal capillary. This wall is fenestrated, allowing easy movement of substances out of the capillary (in contrast to capillary walls elsewhere in the brain, which, as discussed in Chapter 6, are tightly sealed). The second layer, consisting of scattered pial cells and some collagen, is fragmentary. The third layer, derived from the same layer of cells that forms the ependymal lining of the ventricles, is the choroid epithelium. The choroid epithelial cells look as though they are specialized for secretion because they have many basal infoldings, numerous microvilli on the side facing the CSF, and abundant mitochondria. In addition, adjacent cells are connected to one another by arrays of tight junctions that occlude the extracellular space between them. As in the case of the arachnoid barrier layer discussed in Chapter 4, these junctions help limit the movement of substances across the choroid epithelium; some ions are able to diffuse across these tight junctions, but peptides and other larger molecules are blocked.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree