Video–EEG Equipment
Most epilepsy centers use digital video–EEG acquisition systems for video–EEG monitoring. Digital video allows viewing of recorded events remote from the video–EEG monitoring area, provided the hardware and network infrastructure available is adequate to meet the high-volume data stream demands. The video camera selected should have low-light recording capabilities in order to allow the capture of nocturnal events. Cameras selected should also have autofocus functionality and remote control capabilities for camera angle and zoom so as to enable technical staff to acquire optimum video during an event.
The advantages of digital EEG data over analog are significant. Digital EEG data lends itself to postacquisition processing, which is not possible with analog EEG. Spike and seizure detection algorithms can also be run during digital EEG acquisition, which may help in the detection of abnormalities. Selection of amplifiers that allow local data storage with flash memory during disconnections from the primary network offers additional advantages, particularly in active patients such as young children, and those few patients who may require transport away from the central EEG recording area during evaluation.
Personnel
The presence of appropriately trained and experienced EEG technologists is crucial in order to ensure quality recordings. Twenty-four–hour technician coverage is optimal, as equipment issues can arise at any time potentially affecting several hours of data if not promptly addressed. Continuous observation is also critical in patient safety. Nursing staff familiar with seizure identification and management is also important. Patients undergoing epilepsy monitoring are at risk for falls, seizure-related injuries, aspiration, and cardiorespiratory complications, all of which can be mitigated by prompt nursing intervention (4). Nursing and EEG technology personnel are also crucial to provide clinical testing during seizures for semiologic analysis purposes. Physician coverage must be available to manage prolonged seizure activity or seizure-related cardiorespiratory complications. Twenty-four–hour EEG interpretation should also be available in the event questions arise regarding the EEG or clinical status during off hours. Remote access to ongoing video–EEG monitoring data is very helpful for this purpose.
SEIZURE PROVOCATION, PATIENT MANAGEMENT, AND SAFETY CONSIDERATIONS
The goal of presurgical video–EEG monitoring is to record a complete sample of a patient’s seizures in order to clarify the region or regions of seizure onset. Sleep deprivation, photic stimulation, hyperventilation, exercise, and supervised medication withdrawal are commonly used to increase the yield of recorded seizures. Drug withdrawal should only be performed in a setting in which real-time seizure detection and medical intervention are available.
Supervised Medication Withdrawal
Medication withdrawal should be individualized for each patient, balancing the need to record a sufficient number of seizures and the risks based on the patient’s individual seizure history. Starting medication withdrawal prior to admission is not generally advisable given the risks. A common approach is to reduce the dose of one medication by 33% to 50% on the first monitoring day and then to continue reducing the dosages of one or more drugs at a similar rate on successive days until a sufficient number of seizures have been recorded. Individual patient attributes should be kept in mind when strategizing medication withdrawal for epilepsy monitoring purposes. For example, withdrawal may not be necessary at all in patients with a high seizure frequency on full medication therapy. Conversely, some patients with long seizure-free intervals may require a more abrupt withdrawal schedule in order to achieve the monitoring goals. Also, psychiatric difficulties may arise when withdrawing certain antiepileptic drugs with mood stabilizing properties such as valproate, topiramate, carbamazepine, and lamotrigine. Once a tapering plan is decided, it is important to clearly communicate the schedule and goals to the team so that medications are resumed as soon as the objectives have been met, even if this occurs after hours.
Patient Care, Monitoring, and Planning
A standardized rescue plan should be established in the monitoring unit in order to minimize treatment delay in acute seizure emergencies. This rescue plan should include objective criteria for treatment initiation, such as a number of seizures over a defined time period, or for seizures lasting beyond a defined maximal seizure duration. Other criteria for intervention may include the occurrence of a generalized seizure in a patient without a prior history or the emergence of agitation in a patient. While protocols are useful, customization to meet the unique needs of individual patients are often necessary. Creation of an admission order set for the epilepsy monitoring unit containing the standard protocol is advised to ensure that rescue plan orders are put in place at admission. All health care team members need to be educated as to the unit’s standard rescue plan. Immediate access to the patient’s records is required, and regular updates as to the goals of admission need to be documented clearly and communicated to the team and handed off to cross-covering personnel so that once the goals of monitoring have been achieved, antiepileptic medication can be resumed.
Rescue plans typically entail use of intravenous (IV) medications as a component. It is important that IV access be secured in all patients undergoing medication withdrawal upon admission and that processes be in place to monitor IV viability so that it can be relied on in the event of an emergency. It is critical to inquire specifically about drug allergies and significant nonallergic idiosyncratic reactions to potential rescue medications at admission so that needs for deviation from the standard rescue plan can be identified early. IV lorazepam or diazepam at subanesthetic levels are typically used in epilepsy monitoring rescue plans. IV fosphenytoin can also be considered, but requires EKG and blood pressure monitoring and should only be used in a setting where cardiac arrhythmia and hypotension can be acutely managed if detected.
Given the risk of cardiac arrhythmia during seizures (5), continuous EKG monitoring should be performed during video–EEG monitoring. Continuous pulse oximetry should also be used as apnea can complicate seizures. Twenty-four–hour physician availability is necessary to handle any acute situations that may arise during hospitalization. ICU and supportive services such as a rapid response team should be available in the event of the need for acute airway management and resuscitation.
Uniform policies for ambulation and activity should be established and made clear to patients upon admission due to the risk of seizure-related falls and injury. Sharp corners in the seizure-monitoring environment should be minimized. An unobstructed path to the patient bed needs to be ensured so that staff can attend to the patient in a timely manner. Bathroom fixtures pose safety risks, and accommodations need to be considered to minimize the potential for injury in the event a seizure occurs there. Side rails with pads are reasonable to help prevent patients from falling out of bed during a seizure; however, they can pose an unintended risk in some cases, particularly those with hypermotor semiology.
Risks of Video–EEG Monitoring
Medication withdrawal can result in first-time generalized tonic–clonic seizures and other new-onset seizure types in patients without a history of similar seizures, and this can be traumatic for the patient and family. Tongue bite wounds are not infrequent but rarely require treatment beyond conservative measures. Other injuries that can occur in association with video–EEG monitoring include vertebral compression fractures and shoulder dislocations. Caution is required when reducing antiepileptic medications in patients with a previous history of shoulder dislocation and in patients with an established diagnosis of osteopenia. Seizure-related falls can lead to subdural hematoma and skull fractures. Exercise modalities that do not require an upright posture should also be considered. If treadmills and exercise bicycles are used, a nurse or aide need to be present to help prevent seizure-related falls.
Postictal psychosis can arise in the EEG monitoring setting (6). Patients with a prior history of postictal psychosis are at higher risk, but psychosis can occur for the first time in the EEG monitoring setting. Clinical presentation may include aggressiveness and combative behavior, posing a risk to the staff. An action plan needs to be anticipated in patients deemed at risk based on prior history and availability, and close collaboration with psychiatric services is crucial in these cases.
Dismissal from the Video–EEG Monitoring Unit
Patient stability needs to be assured prior to dismissal. Patients undergoing EEG monitoring often have been subjected to significant changes in antiepileptic medication therapy over a short period of time, which may predispose them to further seizures following discharge. Typically, the patient’s usual medication regimen should be resumed 24 hours prior to dismissal. In some cases, it may be prudent to reload patients with parenteral or oral loading doses of their maintenance therapy in anticipation of dismissal. In particularly unstable patients, one should consider obtaining serum levels in order to ensure achievement of therapeutic drug concentrations prior to discharge. It is also advisable to ensure appropriate supervision for the next 1 to 2 days following dismissal by family or other appropriate caretakers.
VIDEO–EEG AND LOCALIZATION OF THE EPILEPTOGENIC ZONE
Clinical Localization: Ictal Semiology
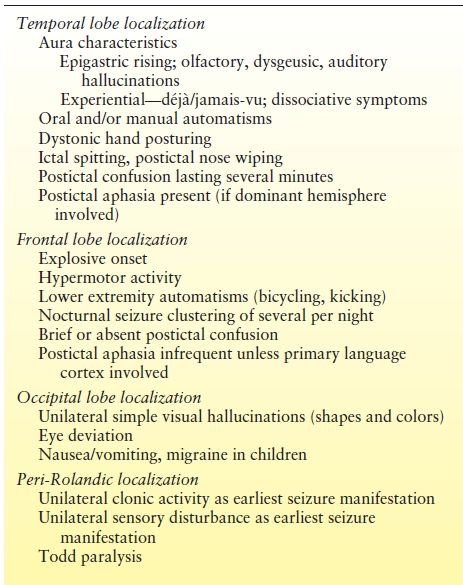
Analysis of ictal semiology can provide valuable localizing information complementary to the ictal EEG (7). Concordance between the ictal semiology and other localizing data helps strengthen hypotheses regarding localization, while discordance should raise concerns. Similarly, the presence of multiple semiologies suggests the possibility of multiple seizure foci, which may influence the prospects for surgical success. Lateralizing and lobar localizing signs are not present in all seizures in all patients, but are specific when present. In one study, lateralizing signs were present in 46% of recorded seizures and 78% of patients (8). Lateralization by ictal semiology was correct in 78% of a population with excellent surgery outcome in two studies (8,9). In terms of lobar localization, temporal versus frontal lobar localization could be correctly determined in 76% of patients by semiologic analysis of a cohort of patients with Engel class I outcome (10). Lateralizing and localizing signs of significance in epilepsy surgery patients are summarized in Tables 74.2 and Table 74.3 and discussed further below.
Table 74.2 Ictal Semiology: Contralateral and Ipsilateral Lateralizing Signs
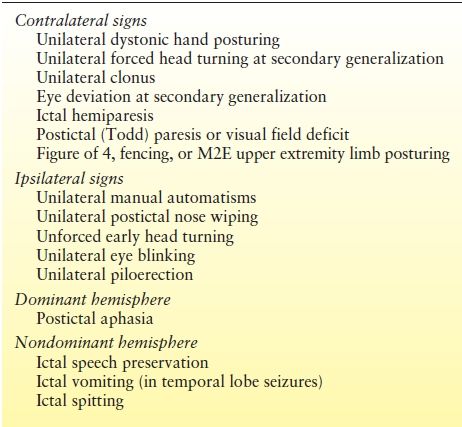
Table 74.3 Ictal Semiology: Lobar Localizing Signs
A number of principles need to be kept in mind when analyzing ictal semiology. First, clinical seizure manifestations are influenced by the propagation of seizure activity from one cortical region to another, which can lead to false localization. For example, aphasia may occur in patients with seizures of nondominant temporal origin following spread to the dominant hemisphere. Second, while the specificity of some of the semiologic signs approach 90%, the sensitivity is lower, and localizing clinical signs may be absent in some patients (8,10). Third, seizures arising in functionally silent regions may not show clinical manifestations until spread to eloquent cortex has occurred, which might falsely suggest seizure origin in the region of propagation. Finally, some regions of the brain lead primarily to subjective perceptual changes that are not appreciable on review of video data due to the absence of a motor or behavioral correlate.
Lateralizing Signs
Some clinical signs are primarily of lateralizing value. These are summarized in Table 74.2 and are discussed in more detail in the following section. Select lateralizing signs are illustrated in Figures 74.1 through 74.3.

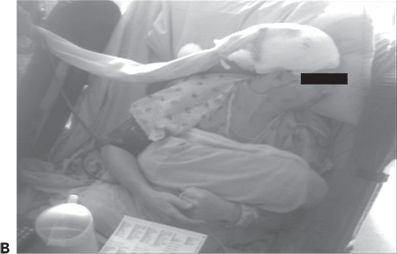
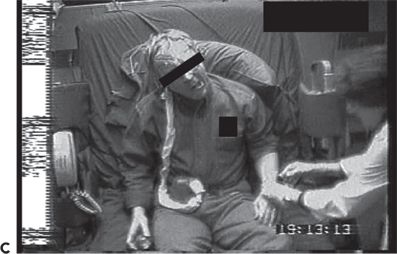


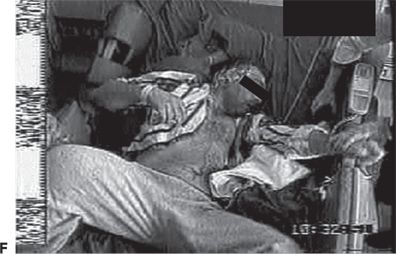

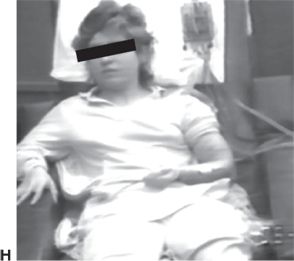
Figure 74.1. Lateralizing signs in patients with partial seizures. A: Unilateral dystonic hand posturing on the left and unforced head turn to the right during a right temporal seizure in a patient with right mesial temporal sclerosis. B: Forced head turning to the left during progression to a secondary generalized seizure in a seizure of right temporal origin secondary to mesial temporal sclerosis. C: Left facial contracture and clonus during a seizure of right frontocentral onset in a patient with a right peri-Rolandic cortical dysplasia. D: Unilateral postictal nose wiping involving the ipsilateral hand in a patient with right temporal seizures. E: “M2E” posturing in a patient with right temporal seizures of unknown etiology. F: “Fencing” posture in a patient with a secondary generalized seizure of right temporal neocortical onset. G: “Figure of 4” posturing with left upper extremity extended during a secondary generalized seizure of right temporal origin. H: Ictal paresis involving the left upper extremity during a right parietal seizure of unknown etiology.
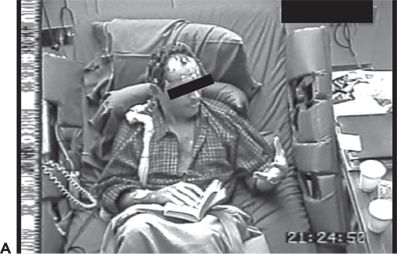
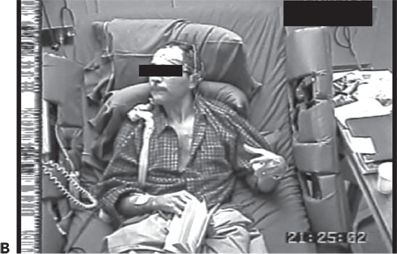
Figure 74.2. Semiologic signs of lobar localizing significance in partial epilepsy. A: Oral automatisms and “regarding the hand” (left hand in this case) in a patient with temporal lobe epilepsy. B: Dystonic left hand posturing, continued oral automatisms, and nonforced right head turn during a right temporal lobe seizure.
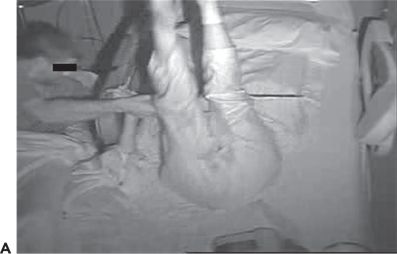
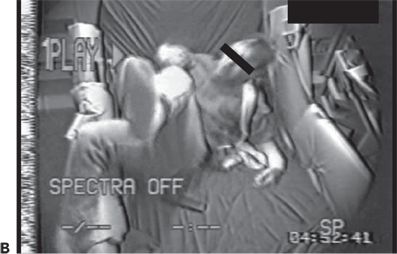
Figure 74.3. A and B: Complex lower extremity automatisms in two patients with frontal lobe epilepsy.
Ictal Speech Preservation and Aphasia.
Ictal speech preservation is highly suggestive of nondominant lateralization in temporal lobe seizures, while ictal aphasia suggests involvement of the dominant hemisphere, frequently of the dominant temporal lobe (11). Ictal aphasia may also occur in nondominant temporal lobe seizures following contralateral propagation. Measuring the time to speech recovery can help in such cases. In one prospective study, recovery of speech within 1 minute of seizure onset was associated with nondominant lateralization (12). Ictal aphasia is less common in dominant hemisphere extratemporal seizures, except for those seizures arising in close proximity to the operculum. When assessing speech during seizure activity, it is important to make sure that any detected speech alteration is not primarily due to orolingual motor impairment, for example, secondary to tonic, clonic, or atonic involvement of the tongue or larynx, as the localizing implications are different.
Unilateral Dystonic Hand Posturing.
Unilateral dystonic hand posturing is associated with contralateral seizure onset (13). This sign is common in temporal lobe seizures and thought to be due to seizure propagation to neighboring basal ganglia. Figure 74.1A shows a patient with unilateral dystonic hand posturing.
Ipsilateral Unilateral Manual Automatisms.
Unilateral manual automatisms are of lateralizing significance primarily when seen in association with unilateral dystonic posturing affecting the contralateral hand (13). When present, unilateral automatisms usually involve the ipsilateral hand. Unilateral automatisms can be mistaken for unilateral upper extremity clonic activity. Distinguishing unilateral automatisms from clonus is important as the lateralizing implications are opposite.
Unilateral Forced Head Turning (Version) at Secondary Generalization.
Forced head turning during transformation from a partial to a secondary generalized seizure typically occurs in the direction contralateral to the hemisphere of seizure onset (14). Head-turning movements are considered versive if they are unquestionably forced and involuntary, resulting in sustained or unnatural posturing (15). Figure 74.1B shows an example of forced head turning. Conversely, early nonforced head turning usually occurs ipsilateral to the seizure focus, but this is less reliable than late forced head turning. Figure 74.1A shows ipsilateral nonforced head turning in addition to contralateral dystonic hand posturing.
Unilateral Forced Head Turning (Version) at the End of Secondary Generalization.
Forced head turning at the end of a secondary generalized tonic–clonic seizure is frequently ipsilateral to the side of seizure onset. Spread of the seizure to the contralateral hemisphere during the course of the seizure is responsible for late ipsiversion (16). Therefore, late version, unlike initial version, is frequently ipsilateral and cannot be assumed to indicate seizure onset in the contralateral hemisphere.
Unilateral Facial or Limb Clonic Seizures.
Unilateral clonus lateralizes to the contralateral hemisphere. Unilateral facial clonic seizures can sometimes present on the scalp EEG in the form of asymmetric rhythmic muscle artifact involving the derivations overlying the affected facial and scalp muscles. Figure 74.1C shows unilateral left facial contraction contralateral to a right frontocentral seizure focus secondary to a right precentral focal cortical dysplasia.
Ictal Vomiting.
Ictal vomiting is an uncommon seizure manifestation that correlates with nondominant lateralization when present in temporal lobe seizures (17), although exceptions with dominant onset exist (18). Ictal vomiting may also present in occipital seizures, in which case it is of less lateralizing significance.
Postictal Nose Wiping.
Nose wiping with one hand following temporal lobe seizures typically involves the ipsilateral hand (19). Postictal nose wiping is more characteristic of temporal as opposed to extratemporal seizures (20). Unilateral nose wiping is illustrated in a patient following a right temporal lobe seizure in Figure 74.1D.
Ictal Spitting.
Ictal spitting is usually associated with nondominant temporal lobe seizures (21). It is thought to be due to hypersalivation secondary to stimulation of the central autonomic network.
Unilateral Piloerection.
This typically occurs ipsilateral to the seizure focus and is usually seen in temporal lobe seizures (22).
M2E, Fencing, and Figure of 4 Posturing.
“M2E” posturing refers to a posture consisting of contralateral shoulder abduction, elbow flexion, and head deviation toward the elevated arm. This is depicted in Figure 74.1E in a patient with right frontal seizures. The “fencing” posture refers to a position assumed during secondary generalization where the contralateral upper extremity is extended, the ipsilateral arm flexed and abducted at the shoulder, and head rotated contralateral to the seizure focus. The hips may be abducted as well. An example is shown in Figure 74.1F. “Figure of 4” posturing refers to a position where the contralateral upper extremity is extended and the ipsilateral upper extremity flexed at the elbow so that it crosses the extended arm giving rise to the shape of the number “4” (23). An example is shown in Figure 74.1G.
Todd’s Paresis and Ictal Paresis.
While relatively uncommon, unilateral postictal Todd paralysis correlates with seizure lateralization to the contralateral hemisphere. Todd paralysis is more common in seizures arising from the peri-Rolandic region, but can be seen in the context of temporal lobe epilepsy. “Ictal paresis” is a rare semiologic manifestation typically occurring contralateral to the seizure focus in patients with extratemporal seizures (24). Ictal paresis can be mistaken for transient ischemic attacks. An example is shown in Figure 74.1H.
Lobar Localization
Semiology can help with lobar localization, particularly in differentiating temporal and extratemporal seizures.
Temporal Localization.
An aura of experiential phenomena such as an out-of-body experience, epigastric rising sensation, and olfactory and dysgeusic hallucinosis suggests temporal localization. Manual and oral automatisms present frequently during temporal lobe seizures, and verbal and nonverbal vocalizations may be present. Cessation of activity at seizure onset is common. Dystonic hand posturing may occur. Figure 74.2A and B show unilateral dystonic hand posturing and oral automatisms in a patient during a right temporal lobe seizure. Temporal lobe seizures typically last 1 to 3 minutes in duration. A postictal confusional period lasting a few to several minutes followed by a desire to sleep is also typical in temporal lobe seizures. However nondominant temporal lobe seizures and those with limited bitemporal involvement may not be associated with a significant postictal period.
Frontal Lobe Seizures.
The clinical presentations of extratemporal frontal lobe seizures are protean. A number of semiologic differences have been identified (10). In contrast to temporal lobe seizures, frontal lobe seizure auras, if present, are usually less descript, at times consisting of vague light-headedness. Frontal seizures are often brief, lasting 1 minute or less, and are sometimes characterized by an explosive onset, with prominent hypermotor activity and complex lower extremity automatisms such as bicycling movements and kicking (Fig. 74.3A and B). Vocalizations, such as the utterance of profanities and screaming, may also occur. In some patients, extratemporal seizures, in particular frontal lobe seizures, occur more frequently out of sleep (25). While nocturnal predominance may be seen in temporal lobe seizures as well, a seizure pattern of multiple brief clusters of seizures occurring exclusively during sleep is more characteristic of frontal lobe seizures. Nongeneralized seizures of frontal origin are often followed by a brief postictal period relative to that of temporal lobe seizures. However, not all frontal lobe seizures behave in the same manner. Some frontal lobe seizures may evolve over prolonged periods of time, and postictal confusion may sometimes be seen. Semiology is particularly important in the process of diagnosing frontal lobe seizures, as the ictal EEG is often nondiagnostic in these patients, particularly in those with seizures of supplementary motor or orbital frontal onset (26).
EEG Localization in Video–EEG Monitoring
Interictal and ictal EEG analysis are essential in the presurgical evaluation. An understanding of the localizing patterns seen in partial epilepsy and limitations of ictal EEG localization are essential.
A minimum of 20 scalp EEG channels should be used when performing prolonged video–EEG monitoring. Midline, right, and left parasagittal and right and left temporal head electrodes should be utilized. Additional inferior temporal electrodes should be considered in patients where a temporal lobe focus is suspected. Nasopharyngeal, foramen ovale, and transsphenoidal electrodes have been advocated by some to improve the sensitivity and specificity of ictal EEG localization, particularly in patients with mesial temporal seizures.
Interictal Epileptiform Abnormalities
Although the ictal EEG receives the most scrutiny in video–EEG monitoring, the interictal recording is also of localizing value. The interictal background may contain focal slowing or suppression over the epileptogenic region, which can help localize areas of brain dysfunction relevant to the patient’s seizures. Also, the presence of interictal epileptiform abnormalities beyond the boundaries of the epileptogenic zone or contralateral to the suspected focus may influence surgical prognostication (27). Generalized interictal epileptiform activity may be seen in addition to focal abnormalities in some patients with focal epilepsy, suggesting a mixed seizure disorder. Finally, in some cases, the interictal EEG may be of greater localizing value than the ictal EEG, particularly in extratemporal focal epilepsy. Figure 74.4A–D shows focal interictal epileptiform abnormalities in four patients undergoing epilepsy surgery evaluation. In the extratemporal cases shown (see Fig. 74.4B–D), the interictal activity had clearer localizing attributes than the ictal EEG.
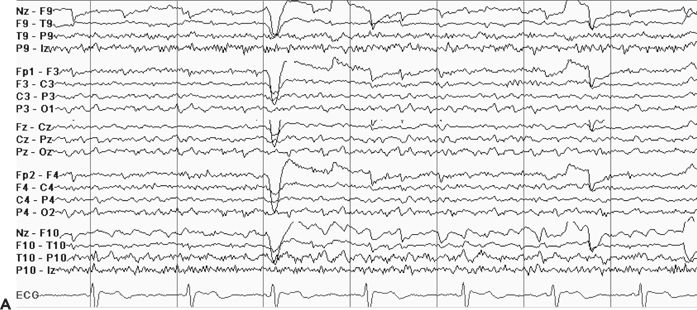
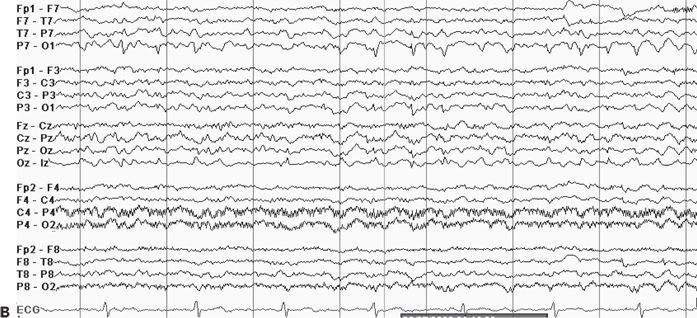
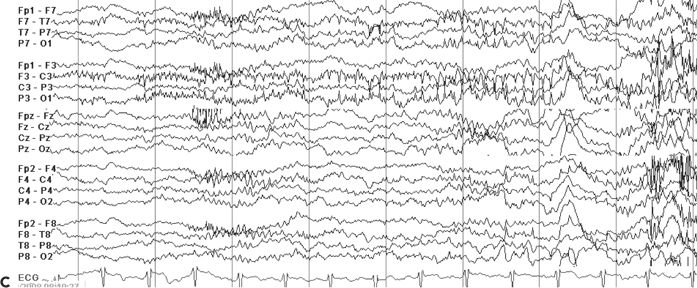
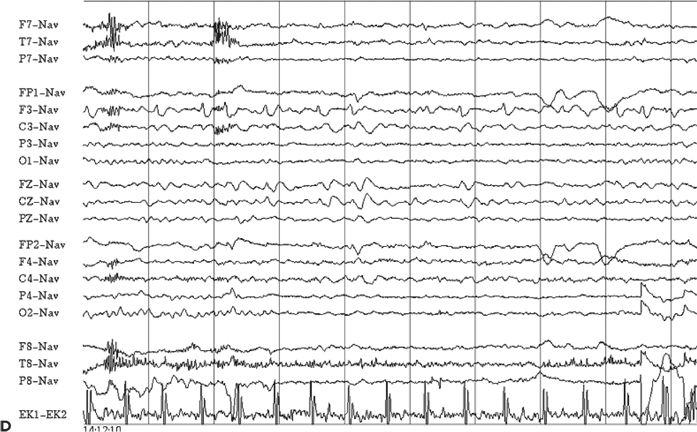
Figure 74.4. Localizing interictal abnormalities in epilepsy surgery patients with partial epilepsy. A: Right temporal sharp waves and temporal intermittent rhythmic delta activity (TIRDA) in a patient with right temporal lobe epilepsy secondary to mesial temporal sclerosis. B: Left occipitoposterior temporal spikes in a patient with a left medial occipital cortical dysplasia. C: Left centroparietotemporal spikes in a patient with a cortical dysplasia localized to the left inferolateral postcentral gyrus region. D: Repetitive left frontal spikes in a patient with nonlesional frontal lobe epilepsy localized with intracranial monitoring to the left dorsolateral frontal region.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








