49 Viral Diseases
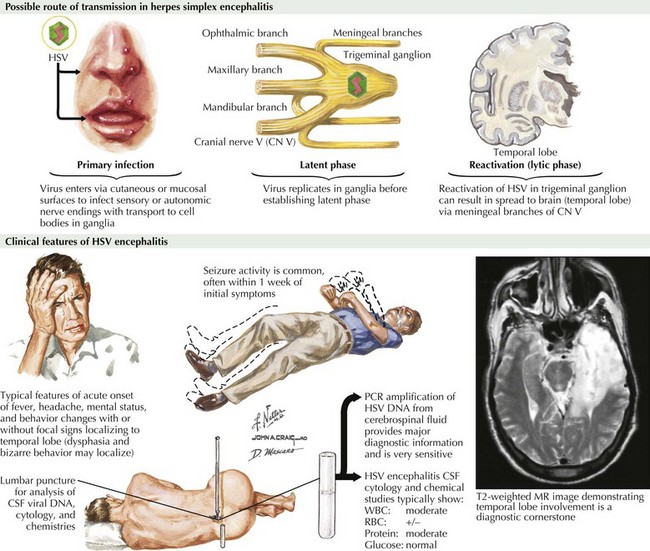
Herpes Simplex Encephalitis
Clinical Presentation
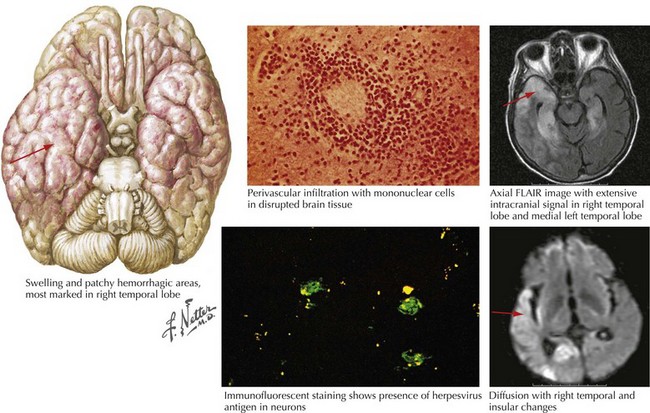
The symptoms and signs of patients with subacute or acute focal encephalitis are generally rather nonspecific, with fever, headache, and altered consciousness being the most common. Focal manifestations often include seizures, typically complex partial in character because of the temporal lobe’s predisposition to develop a herpes infection. It is common for these patients to also develop language difficulties, personality changes, hemiparesis, ataxia, cranial nerve defects, and papilledema (Fig. 49-1). The differential diagnosis includes stroke, brain tumors, other viral encephalitides, bacterial abscesses, tuberculosis, cryptococcal infections, and toxoplasmosis.
Diagnosis
For patients with suspected encephalitis, the initial diagnostic studies must include a CT scan (to rule out a mass effect) and then immediate CSF examination. CT scan results are abnormal in 50% of cases early on and usually demonstrate localized edema, low-density lesions, mass effects, contrast enhancements, or hemorrhage. MRI and EEG may be subsequently obtained for further confirmation. These usually demonstrate major temporal lobe damage (Fig. 49-2); however, a normal study does not exclude an HSE diagnosis. If such does occur, it is often wise to repeat the study within a few days, particularly if the patient continues to be confused.
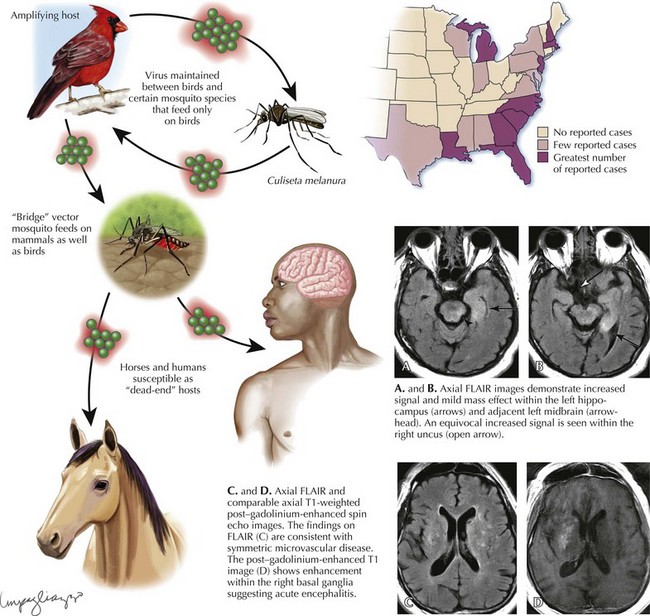
Eastern Equine Encephalitis
Diagnosis
Laboratory diagnosis of EEE virus infection is based on serology, especially IgM testing of serum and CSF, and neutralizing antibody testing of acute- and convalescent-phase serum. MRI is the most sensitive imaging modality for diagnosis of EEE (Fig. 49-3). The most commonly affected areas of the central nervous system (CNS) include the basal ganglia (unilateral or asymmetric, with occasional internal capsule involvement) and thalamic nuclei. Other areas include the brain stem (often the midbrain), periventricular white matter, and cortex (most often temporally). Affected areas appear as increased signal intensity on T2-weighted images.