Model
Construct Validity?
Reproducible?
Progressive nigrostriatal degeneration?
Parkinsonian pathology?
Parkinsonian symptoms?
References
Construct validity for idiopathic PD
Within group
Across groups
Striatal DA terminal dysfunction/loss (over weeks)
Terminal loss precedes overt cell loss
Nigral DA neuron loss (≥8 weeks)
End stage nigral DA neuron loss >60%
Lewy-body-like inclusions
Dystrophic neurites
No overt, non-PD pathology
Neuro-inflammation
Motor impairment
Rats
Mice
Non-human primate
α–Synuclein overexpression
Wild type
Yes
Yes
Yes
Yes
Yes
Yes
With high expression
Yes
Yes
Yes
Yes
Yes
A30P
Yes
Yes
Yes
Yes
Yes
Yes
No
Yes
Yes
Yes
No data
No
[60]
A53T
Yes
Yes
Yes
Yes (on limited data)
No (on limited data)
Yes
No
Yes
Yes
Yes
Yes
Yes
[52]
E35K, E46K, E57K
Yes
Not demonstrated
Not demonstrated
No data
No data
No
No
No data
No data
Yes
No data
No data
[40]
S129A
Yes
Not demonstrated
Yes
Yes
Yes
Yes
Yes
Yes
Yes
No
No data
No data
LRRK-2 overexpression
Wild type
Yes
Not demonstrated
Yes
No
No
No
No
No
Yes?
No
No
No
[75]
[76]
G2019S
Yes
Not demonstrated
Yes
Yes
No (on limited data)
Yes
No
No
Yes?
No
Yes (on limited data)
No
[75]
[76]
D1994A
Yes
Not demonstrated
Not demonstrated
No
No
No
No
No data
No data
No
No data
No
[76]
G2019S + D1994A
Yes
Not demonstrated
Not demonstrated
No
No
No
No
No data
No data
No
No data
No
[76]
TH shRNA
No
Not demonstrated
Yes
Yes, dysfunction only
No
No
No
No
No
No
No data
Yes
[87]
[86]
Parkin subtrates
CD-Crel1
Yes
Not demonstrated
Not demonstrated
Yes
Yes
Yes
No
No data
No data
No
No
No data
[81]
Pael-R
Yes
Not demonstrated
Yes
Yes
No
Yes
Not quite
No
No
No
No
Yes (stepping test only)
[83]
[82]
hu p38/JTV
Yes
Not demonstrated
Not demonstrated
No data
No data
No
No
No
No
No
No data
No data
[84]
hu wt synphilin-1
Yes
Not demonstrated
Not demonstrated
No data
No data
No
No
No
No data
No
No data
No data
[85]
hu wt synphilin 1 on A30Pα‑synbackground
Yes
Not demonstrated
Not demonstrated
No data
No data
No
No
Yes
No data
No
No data
No data
[85]
hu R621C synphilin-1
Yes
Not demonstrated
Not demonstrated
No data
No data
No
No
No
No data
No
No data
No data
[85]
hu R621C synphilin-1 on A30Pα-synbackground
Yes
Not demonstrated
Not demonstrated
No data
No data
No
No
Yes
No data
No
No data
No data
[85]
PINK1shRNA
Yes
Not demonstrated
Not demonstrated
No
No
No
No
No
No
No
No data
No data
[90]
3.1 α-Synuclein Overexpression
Mutations in the gene encoding α-syn , SNCA, have been definitively linked to familial PD [27]. Increases in overall α-syn expression by duplications or triplications of SNCA and single nucleotide mutations (viz., A30P, A53T and E46K) within SNCA are associated with development of PD and an earlier onset of disease symptoms [27–32]. In addition, the primary component of Lewy bodies is aggregated α-syn [33]; hence, targeted overexpression of wild-type or mutated α-syn to the adult, nonhuman nigrostriatal system was the first viral vector-based approach to model PD [34–36]. Since that time, gene transfer of α-syn to the SN has been the most extensively studied with numerous reports in mice, rats and nonhuman primates and using both adeno-associated virus es (AAV ) and lentiviral (LV) vector constructs [34–65]. Over time, as technological advances allow for higher vector titers to be achieved and the identification of more efficient promoters, viral vector-mediated overexpression of α-syn has become more consistent and yielded a greater magnitude of effects. Present use of AAV or LV to overexpress α-syn recapitulates several components of PD neuropathology, including: (a) early striatal terminal dysfunction [62, 66], (b) progressive loss of striatal dopaminergic terminals, (c) progressive loss of dopaminergic neurons of the SNc following loss of terminals, (d) Lewy body -like inclusions containing α-syn, (e) dystrophic neurites resembling Lewy neurites [35, 41, 46, 67] and (f) neuroinflammation [44, 51, 62, 68]. In addition, viral vector-mediated α-syn overexpression results in PD-like motor symptoms that correlate with an approximate 50 % loss of striatal DA as observed in PD patients [47, 49].
In our own laboratory, we have characterized the degeneration, pathology and behavioral phenotype induced by intranigral injections of recombinant AAV serotype 2/5 (AAV2/5) in which expression of the human wild-type α-syn transgene is driven by the chicken beta actin/cytomegalovirus (CβA/CMV) enhancer-promoter hybrid that results in efficient gene expression in neurons [49] (see Fig. 1). This model results in: (a) transduction of the nigrostriatal system with human wild-type α-syn, (b) 60 % nigral DA neuron loss and 40 % reduction in striatal TH immunoreactivity 8 weeks post injection, (c) marked microgliosis in the SN associated with α-syn overexpression and (d) significant impairment in contralateral forelimb use 8 weeks post injection. Lastly, nigral neuron degeneration correlates with α-syn expression levels and can be adjusted by the investigator through altering the vector titer or construct [49], offering a methodological advantage.
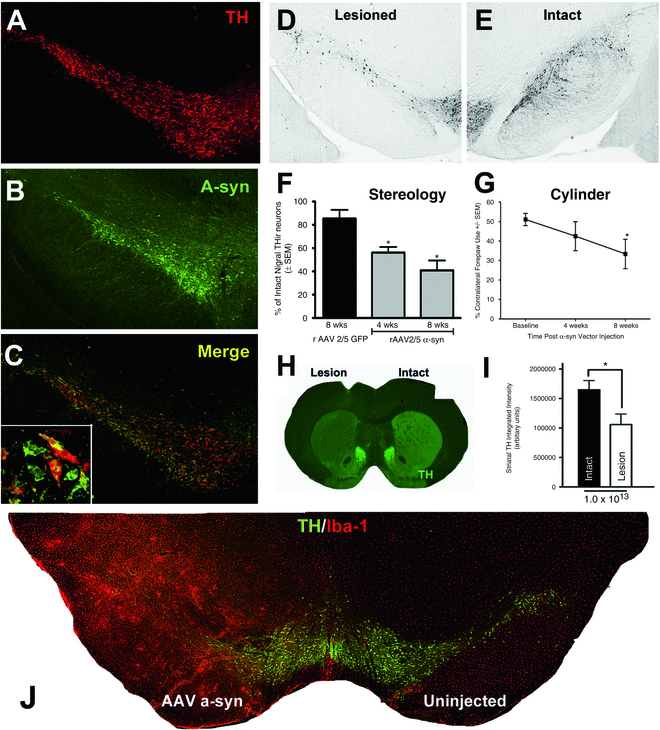

Fig. 1
Overexpression of α-syn uclein in the rat nigrostriatal system via AAV2/5 results in nigral degeneration, contralateral forelimb impairment and microgliosis. Intranigral injections of AAV2/5 α-syn (2 × 2 μl of 1 × 1013 vg/ml, AP −5.3, ML +2.0 mm, DV −7.2 mm; AP −6.0 mm, ML +2.0 mm, DV −7.2 mm) were made to young-adult, male, Sprague Dawley rats as described previously [49]. (a–c) Coexpression of human, wild-type α-syn (green) in TH immunoreactive (THir, red) neurons within the SNc at 2 weeks following injections, prior to degeneration. (d) Degeneration of THir neurons of the SNc at 8 weeks after AAV2/5 α-syn injections compared to the uninjected, contralateral SN (e). (f) Stereological assessment revealed that after 4 weeks post α-syn vector injections, there is an approximate 40 % decrease in THir neurons in the SNc that progresses to about 60 % at 8 weeks (*p < 0.05 compared to green fluorescent protein (GFP) control). (g) Significant deficits in contralateral forelimb use are observed 8 weeks after AAV2/5 α-syn injections (*p < 0.05 compared to baseline). (h) Partial dopaminergic striatal denervation ipsilateral to α-syn overexpression at 8 weeks visualized using near infrared immunofluorescence and quantified in i (*p < 0.05 compared to baseline). (j) Microgliosis is associated with α-syn overexpression and nigral degeneration. THir SNc neurons (green) and ionized calcium binding adaptor molecule 1 (Iba-1, microglia-specific, red) immunofluorescence reveals marked microgliosis 8 weeks after AAV2/5 α-syn injections. Adapted from ref. [49]
3.2 LRRK-2 Overexpression
LRRK-2, a protein without a definitively known function or substrate, is 2527 amino acids long and located in the cytoplasm (reviewed in ref. 69). Several different mutations in the LRKK2 gene have been linked to the development of PD in an autosomal dominant inheritance pattern [70–73]. These cases generally include the formation of α-syn immunoreactive Lewy bodies [74]. They also result in the hallmark loss of striatal DA and nigral neuron degeneration found in idiopathic PD. Viral vector -mediated overexpression of LRRK-2 has been used far less extensively (i.e., in only two laboratories) than α-syn-based models [75, 76]. This is primarily due to the prohibitively large size of the LRRK-2 coding sequence, precluding its use in standard vectors such as AAV or lentivirus. Loss of striatal fibers and nigral neurons is of too low a magnitude to model anything more than very early PD. Furthermore, these models produce LRRK-2 complexes that are α-syn deficient and do not resemble Lewy bodies. On the other hand, dystrophic neurites are observed in the striatum with immunoreactivity for the pathological phospho-tau epitope (pSer202/pThr205), but α-syn immunoreactivity is not reported. No data exist on whether motor impairments are present; however, the low magnitude of striatal DA loss suggests motor deficits are unlikely to exist.
3.3 Parkin Substrates
Mutations in the gene for parkin have been linked to an autosomal recessive inheritance pattern of a very early-onset (i.e., juvenile) form of PD [77, 78]. These forms of PD may be better described as parkinsonisms in that they do not show the formation of Lewy bodies, but they do still exhibit loss of nigral DA neurons [79, 80]. Manipulating parkin for modeling PD has proven more difficult for gene therapy approaches since the development of the model requires a complete knockdown of the protein in order to mimic both copies of the gene being mutated in the human condition and to result in pathology. However, since patients with parkin mutations have increased levels of parkin substrates (i.e., parkin is unable to process the increasing supply of substrate), viral vector-mediated overexpression of parkin substrates instead has been used to create a “loss-of-function” paradigm—rather, loss-of-function paradigms, as four parkin substrates have been used with variable results.
In two models overexpressing parkin substrates, CDCrel-1 or Pael-R, a partial loss of striatal DA terminals and concomitant loss of DA is observed, and this loss is progressive on the order of weeks [81–83]. For models overexpressing other parkin substrates, p38/JTV or synphilin, no data on terminal status are available [84, 85]. It is unfortunate that the magnitude of terminal loss matches the loss of nigral DA neurons, suggesting that overt terminal loss does not precede overt loss of perikarya, although this has not been directly examined. Using CDCrel-1 or Pael-R has the advantage over p38/JTV or synphilin in that nigral neuron loss occurs over many weeks proceeding to an eventual loss that corresponds to late-stage disease. Achieving half of the cell loss of these models over a similar time span with p38/JTV or synphilin requires the addition of A30P α-syn expression to create a pro-pathology environment. In these models, A30P α-syn expression lends the advantage of producing some neuropathology, including thioflavin-S positive inclusions. Lastly, the data available do not include behavioral assays to assess motor symptoms, with one exception: Pael-R overexpression will result in contralateral forelimb akinesia in the stepping test, but deficits were not observed in amphetamine- or apomorphine-induced rotations, nor in the cylinder task [83].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






