Zonisamide
Timothy E. Welty
Zonisamide was first synthesized in 1974 in Japan. In the early 1980s, clinical trials of zonisamide were initiated in the United States. Because of an increased risk for nephrolithiasis in patients receiving active drug, further development in the United States was halted. However, zonisamide continued to be developed in Japan, with the agent receiving marketing approval in that country in 1989. Following approval in Japan and the development of improved treatments for nephrolithiasis, additional studies in Europe and the United States were initiated, with marketing approval granted in the United States in 2000.
As a result of the gap in development of ZNS in Western nations, much information on the agent is from the Japanese experience. Language barriers have limited access to some of this information, and the available data may not always be applicable to populations and ethnic groups outside of Japan. Nevertheless, ZNS is an effective and safe antiepileptic drug (AED) that appears to have broad activity in patients with various seizure types and epilepsy syndromes.
CHEMISTRY
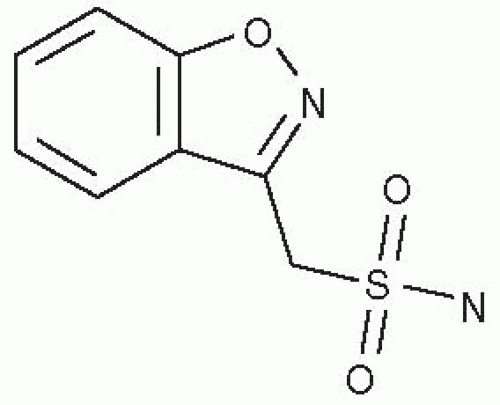
ZNS is classified as a sulfonamide AED that is a 1,2 benzisoxazole derivative. It is the first compound from this group of chemicals to be developed as an AED. ZNS is unrelated chemically to other AEDs (Fig. 62.1). The agent is moderately soluble in water (0.8 mg/mL) and has a pKa of 10.2. ZNS is a white powder; it has a molecular weight of 212.23.
MECHANISM OF ACTION
Several pharmacologic effects of ZNS may be responsible for its activity as an AED. Some of these activities may make ZNS useful in the treatment of other neurologic disorders as well. Results from numerous studies (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19) demonstrate the most likely mechanism of action of ZNS to be via blockade of T-type calcium channels, inhibition of slow sodium channels, and possibly inhibition of glutamate release. In animal models of epilepsy, ZNS demonstrates activity that indicates its possible effectiveness as a broad-spectrum AED. Besides its antiepileptic activity, ZNS also has some effect as a neuroprotective agent in the treatment of ischemia (20).
PHARMACOKINETICS
Absorption
ZNS is rapidly absorbed following oral administration, with maximum concentrations achieved within 2 to 5 hours (21). The absolute bioavailability in humans is unknown because of lack of a parenteral product. Nagatomi and colleagues found the absolute bioavailability of orally administered ZNS to be 81% in rats (22). In the same study, the bioavailability of ZNS in a rectal preparation was 96%. ZNS is metabolized by the cytochrome P450 isozyme 3A4 (CYP3A4) (23). The presence of CYP3A4 in the intestine may account for the decreased bioavailability of the oral preparation.
Distribution and Protein Binding
Like many sulfonamide drugs, ZNS has a dose-dependent decrease in volume of distribution (Vd) (24). The Vd is 1.8 L/kg for a 200-mg dose and 1.2 L/kg for an 800-mg dose. Saturable binding to erythrocytes, particularly intracellular carbonic anhydrase, is the most likely explanation for this phenomenon (25, 26, 27). Additionally, 40% to 60% of ZNS is bound to plasma proteins, especially albumin (27,28).
Therefore, ZNS is concentrated in erythrocytes, not in plasma. With saturable binding to erythrocytes, the whole blood ZNS concentration is nonlinear as the dosage increases. However, the plasma ZNS concentration is linear
with increased doses (21). Care must be taken in laboratory analysis and interpretation of ZNS concentrations. Results should be identified as obtained from either whole blood or plasma.
with increased doses (21). Care must be taken in laboratory analysis and interpretation of ZNS concentrations. Results should be identified as obtained from either whole blood or plasma.
Metabolism and Clearance
Following oral administration, the half-life (t1/2) of ZNS is estimated at 50 to 69 hours (21,29). Total clearance (Cl) following single and repeated oral doses is 0.6 to 0.71 L per hour (29). Less than 30% of ZNS is eliminated unchanged in the urine and most of the drug undergoes extensive hepatic metabolism (30). The relatively long t1/2 and slow clearance allow for once-daily ZNS dosing.
Early studies of the pharmacokinetics of ZNS suggested that concentrations increased in a nonlinear relationship to dose (24,31). Following administration of an 800-mg dose, ZNS clearance was 22% lower than clearance estimates following 200-mg and 400-mg doses. Clearance estimates at steady state, with doses ranging from 400 to 1200 mg per day, were 40% lower than those seen following a single 400-mg dose (21,32). In a study by Wilensky and associates, although steady-state ZNS concentrations were found to be higher than predicted from single-dose data, steady-state plasma concentrations increased in a linear relationship to daily dose (33). These observations were considered to be related to the saturable, preferential binding of ZNS to erythrocytes. However, an analysis of ZNS doses and concentration in children using a non-linear mixed effects model and population pharmacokinetic methodology demonstrated dose-dependent, Michaelis-Menten pharmacokinetics of zonisamide, with a mean maximum flow velocity (Vmax) of 27.6 mg/day/kg and an affinity constant (Km) of 45.9 μg/mL (34). Because the Vmax is well above the typical range of daily ZNS doses, it is unlikely that the nonlinear nature of ZNS clearance will profoundly impact clinical practice.
The major metabolite of ZNS is 2-sulfamoylacetylphenol (SMAP), formed under anaerobic conditions by liver microsomal enzymes (23,35,36). The formation of SMAP appears to be primarily through CYP3A4 (23,35). As reported in the aforementioned studies (23,35), the metabolism of ZNS to SMAP was inhibited by cimetidine and ketoconazole, which are both known CYP3A4 inhibitors. ZNS is metabolized to a much lesser extent by CYP2C19 and CYP3A5 (37).
Plasma Concentrations and Dosing
The manufacturer’s recommended dose for adults is 200 to 400 mg per day, but doses of 600 mg per day have been used in clinical trials (38). Doses >400 mg have not consistently been associated with increased efficacy. The recommended doses of ZNS are typically associated with steady-state concentrations of 10 to 30 μg/mL (29,34). However, a relationship between concentration and response has not been established. Some investigators have suggested that concentrations >30 μg/mL are associated with increased adverse effects (24,33). Therefore, it may be advisable to maintain ZNS at concentrations <30 μg/mL. The pharmacokinetics and dosing of ZNS are summarized in Table 62.1.
Special Populations
Pediatrics
No formal pharmacokinetic studies have been conducted in children. In a study of ZNS for infantile spasms by Suzuki and colleagues, daily doses of 4 to 5 mg/kg yielded plasma concentrations of 5.2 to 16.3 μg/mL (39). Additional work by the same investigators substantiated these findings, with ZNS doses of 4 to 12 mg/kg per day producing plasma concentrations of 5.2 to 30 μg/mL (40). Table 62.2 summarizes typical mean plasma concentrations related to dose and age. A comparison of pharmacokinetic parameters derived from population data in children and adults shows a similar Vd but more rapid clearance of ZNS in children (34,41). Thus, children appear to require larger doses of zonisamide, based on body weight, to achieve plasma concentrations similar to those seen in adults (42).
Three case reports have provided some documentation regarding transfer of ZNS across the placenta and into
breast milk. Kawada and associates measured ZNS concentrations in umbilical cord blood, infant blood, and maternal blood in two infants born to mothers taking ZNS for the treatment of epilepsy (43). In these infants, ZNS concentrations were 92% of those in maternal blood. The investigators also measured ZNS concentrations in the breast milk of these mothers, showing these concentrations to be 41% to 57% of maternal plasma concentrations. In a separate case evaluating ZNS concentrations in breast milk up to 30 days postpartum, Shimoyama and colleagues observed breast milk concentrations that ranged from 81% to 100% of maternal plasma concentrations (44). It appears that ZNS readily crosses the placenta. ZNS also appears in breast milk at concentrations similar to maternal plasma concentrations. No clinically important adverse effects related to ZNS were documented in these case reports.
breast milk. Kawada and associates measured ZNS concentrations in umbilical cord blood, infant blood, and maternal blood in two infants born to mothers taking ZNS for the treatment of epilepsy (43). In these infants, ZNS concentrations were 92% of those in maternal blood. The investigators also measured ZNS concentrations in the breast milk of these mothers, showing these concentrations to be 41% to 57% of maternal plasma concentrations. In a separate case evaluating ZNS concentrations in breast milk up to 30 days postpartum, Shimoyama and colleagues observed breast milk concentrations that ranged from 81% to 100% of maternal plasma concentrations (44). It appears that ZNS readily crosses the placenta. ZNS also appears in breast milk at concentrations similar to maternal plasma concentrations. No clinically important adverse effects related to ZNS were documented in these case reports.
TABLE 62.1 SUMMARY OF ZNS PHARMACOKINETICS AND DOSING | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
TABLE 62.2 MEAN ZNS PLASMA CONCENTRATIONS RELATED TO AGE AND DAILY DOSE | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree