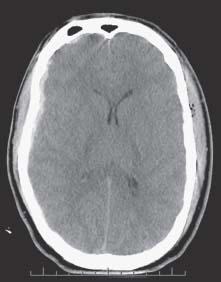
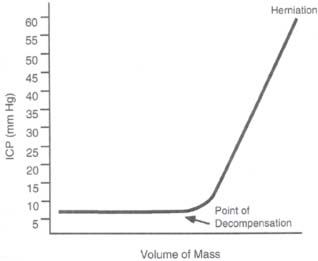
Case 50 Intracranial Pressure Management Abdulrazag Ajlan and Judith Marcoux Fig. 50.1 A brain computed tomography scan showing a small right subdural hematoma with a midline shift of 4 mm. There is diffuse brain swelling and small ventricles. Fig. 50.2 The brain can compensate within a limited range of pressure. After the compensatory mechanisms fail, the intracranial pressure will increase dramatically.
 Clinical Presentation
Clinical Presentation
 Questions
Questions
 Answers
Answers
50 Intracranial Pressure Management
Case 50 Intracranial Pressure Management Fig. 50.1 A brain computed tomography scan showing a small right subdural hematoma with a midline shift of 4 mm. There is diffuse brain swelling and small ventricles. Fig. 50.2 The brain can compensate within a limited range of pressure. After the compensatory mechanisms fail, the intracranial pressure will increase dramatically.


 Clinical Presentation
Clinical Presentation
 Questions
Questions
 Answers
Answers
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


