Figure 46-1 Higher power view of sural nerve biopsy, transverse semithin section, stained with toluidine blue demonstrating loss of myelinated axons, thinly myelinated axons, a circumferentially oriented Schwann cell profile (nascent onion bulb; arrow) (400×).
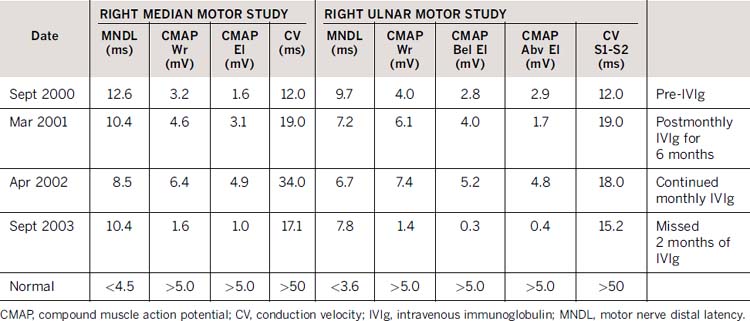
An initial course of prednisone resulted in mild improvement but was stopped because the patient became aggressive and euphoric. Azathioprine was associated with persistent muscle aches, fever and nausea that resolved with discontinuation. He received monthly doses of IVIg with improvement of proximal arm strength and ankle dorsiflexion and eversion. Repeat electrophysiology demonstrated shortening of distal latencies, a prominent reduction in temporal dispersion, and an increase in compound muscle action potential amplitudes following treatment and worsening of these features when IVIg was withheld for longer than 2 months (Table 46-1). Clear deterioration in hand dexterity and overall function occurred with delay of the IVIg treatment and there was improvement with prompt retreatment. The tremor did not respond to treatment and gradually worsened with time.
Table 46-1 Nerve conduction study results demonstrating improved electrophysiology following monthly IVIg administration and deterioration following discontinuation for 2 months
CONCLUSIONS
Distal acquired demyelinating symmetric (DADS) neuropathy typically presents with chronic progressive distal motor and sensory symptoms that are relatively symmetric.1 The majority of patients with DADS will have a monoclonal gammopathy of unknown significance (MGUS) which is typically of the immunoglobulin M IgM subtype. The IgM is specific for MAG in approximately half of the cases.2,3
Testing for anti-MAG antibodies is typically performed in patients with MGUS but some anti-MAG–positive patients may lack the monoclonal spike on serum protein electrophoresis or the MGUS may be discovered at a later time.4 The light chain component is usually κ.2 Anti-MAG antibodies are found in a minority of patients who have Waldenström’s macroglobulinemia, lymphoma, or chronic lymphocytic leukemia.3
Tremor associated with anti-MAG neuropathy is well described, occurring in more than half of patients. In one long-term follow-up study, 20% of patients suffered functional disability related to tremor.5 The tremor is typically of higher amplitude and lower frequency than tremor associated with other demyelinating neuropathies. Physiologic tremor analysis has suggested that it may represent a distinct neurogenic tremor.6
A randomized, double-blind, placebo-controlled study of IVIg in 22 patients (IgM MGUS demyelinating neuropathy; 11 of the 22 being anti-MAG positive) showed a statistically significant improvement in the Inflammatory Neuropathy Cause and Treatment disability score and several other secondary outcome measures in the IVIg-treated group.7 Clinical trial evidence for the specific benefits of IVIg in anti-MAG polyneuropathy, however, is not available.
This patient demonstrates the presentation of anti-MAG neuropathy with chronic progressive and relatively symmetric distal large fiber predominant sensory and motor symptoms in an adult male patient.8 Electrodiagnostic studies demonstrated the features of primary demyelination, with prominent prolonged distal motor latencies. Subsequent hematologic investigations identified a monoclonal gammopathy, with high titers of anti-MAG antibodies, and evidence of an indolent underlying lymphproliferative disorder. Treatment with IVIg resulted in significant improvement in this patient’s function on both the motor examination, as well as electrophysiology. The most disabling symptom of this patient’s neuropathy however, was tremor that did not improve with immunomodulation, propranolol or gabapentin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








