CHAPTER 133 Acoustic Neuroma
History
In 1777, the first reported pathologic case of an acoustic neuroma (vestibular schwannoma) was made in an autopsy report by Eduard Sandidort.1,2 There are several reports from the early 19th century of patients with signs and symptoms consistent with an acoustic neuroma that were validated on postmortem studies. Lasource in 1810 and Bell in 1830 described the progression of symptoms along with postmortem descriptions of what were probably acoustic neuromas.3–5 Throughout most of the 18th century, attempts at resection of acoustic neuromas were associated with significantly high mortality. The high mortality and high morbidity were certainly associated with technique. Postoperative infections, as with other surgical procedures of the time, were common. Advancements in the understanding of aseptic technique and improved anesthetic skill enhanced patient tolerance of surgery and significantly decreased postoperative morbidity.
Some believe that the first successful removal of an acoustic neuroma was performed in 1894 by Sir Charles Balance.6 However, the case report described a tumor with a broad attachment to the dura on the posterior surface of the petrous portion of the temporal bone. In addition, there was no mention of diminished hearing. The absence of decreased hearing would be uncommon in the case of an acoustic neuroma. Cushing believed that these findings were more consistent with a meningioma.4,5 Others, including Cushing, have attributed the first successful removal of an acoustic neuroma to Thomas Annandale. The patient was a young pregnant woman with right-sided hearing loss who survived surgery and subsequently gave birth.
Survival in early attempts to remove acoustic neuromas, as mentioned, was the exception rather than the rule. Results from early series demonstrated a remarkably high mortality rate, with Borchardt reporting an operative mortality of 72%; Eiselberg, 74%; and Krause, 84%.5,7 These disappointing results were at least in part due to technique. Finger enucleation of the tumor would often avulse the anterior inferior cerebellar artery (AICA) and lead to significant bleeding and brainstem infarction.
Cushing was the most influential in ushering in a new era of acoustic surgery with significantly improved survival. In the early 20th century, Cushing refined surgical technique to reduce mortality significantly from higher than 50% to approximately 11%.4,6 Dandy built on Cushing’s work to further lower operative mortality and focused on gross total resection to limit recurrence.8 The disappointing results of early attempts at tumor resection lead Panse to suggest a translabyrinthine approach to avoid the severe complications seen with early retrosigmoid attempts.5 The translabyrinthine approach would be reintroduced by House in the 1960s as an alternative to the retrosigmoid approach.9
The importance of operative experience was evident in the 1960s. Compilations of surgeons infrequently performing the surgery demonstrated a 31% mortality rate.10 However, even with experienced surgeons such as Olivecrona, who operated on 415 acoustic neuromas between 1931 and 1960, there was a reported overall mortality of 19.7%.11 The mortality rate for large tumors in Olivecrona’s series was approximately 5 times what it was for tumors that were “hazelnut size,” thus establishing a differential between tumor size and outcome that persists today.12 In 1961, House would propose the middle fossa approach for acoustic neuroma surgery as part of his Triological thesis.13 The approach had been made popular for treating vestibular neuronitis and other temporal fossa pathology.
Natural History
Schuknecht found an incidence of occult acoustic neuromas of 0.57%, or 570 per 100,000 temporal bones.6,14 The National Institutes of Health Consensus Statement estimated an incidence of approximately 1 in 100,000 in 1991. However, advances in imaging, including routine use of gadolinium enhancement with magnetic resonance imaging (MRI), would probably place the incidence somewhere between the two. The increased sensitivity of current imaging modalities allows physicians to detect tumors at smaller sizes and also brings up the dilemma of what to do with small tumors in asymptomatic or mildly symptomatic patients.
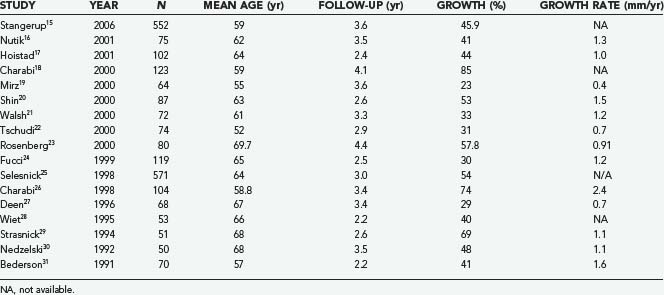
The expected rate of growth plays an important role in determining whether observation will suffice or a surgical intervention is indicated. Including only published series with at least 50 patients, the proportion of tumors that demonstrate growth ranges from 30% to 85% (Table 133-1).15–31 The growth rate also varies from 0.4 to 2.4 mm/yr. Stangerup and colleagues, in a large series of 522 patients with a mean observation time of 3.6 years, found that tumors demonstrating growth did so in the first 5 years after diagnosis. Interestingly, they also found that intrameatal and extrameatal tumors had a statistically different rate of growth, with 17% and 29% of these tumors demonstrating growth within 4 years of diagnosis, respectively.15 These findings suggest that both the time since diagnosis and the location of the tumor with respect to the internal auditory canal (IAC) play a role in the natural history of acoustic tumors. Acoustic neuromas in patients with neurofibromatosis type 2 (NF2) frequently exhibit a distinct growth pattern and are thus often treated as a separate entity. Acoustic neuromas in these patients occur at a younger age and may have a more unpredictable growth rate with more rapid growth the younger the patient at the time of diagnosis.32–35 Slattery and associates found the average growth rate measured in the tumor’s greatest diameter to be 1.3 mm/yr in patients with NF2. Patients who had a family history of NF2 did not experience more rapid growth. This growth rate falls within what is seen in patients without NF2 who harbor acoustic neuromas with gradual, consistent growth.36 NF2 patients often present a challenge because of the increased incidence of bilateral tumors and the younger age at which tumors develop. NF2 tumors present a more complex approach because attempts at maintaining serviceable hearing need to take into account future growth. Obviously, the loss of bilateral serviceable hearing is a more debilitating neurological deficit than unilateral hearing loss.
TABLE 133-1 Percentage of Acoustic Neuromas Demonstrating Growth in Series with at Least 50 Patients
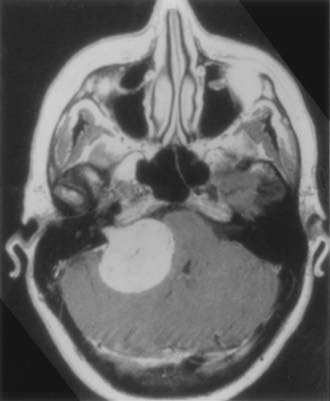
Cystic acoustic tumors are believed by some to have a more aggressive course with more rapid neurological deterioration (Fig. 133-1).37 There is debate within the literature about whether these tumors are associated with greater surgical morbidity, with some authors finding a higher risk for facial nerve palsy with operative treatment of cystic acoustic neuromas.38 However, others report that when corrected for other factors such as size, the surgical morbidity is comparable.39
Histopathologic Characteristics
The terminology “acoustic neuroma” is a misnomer because the cellular makeup of the tumor is actually more consistent with a schwannoma and it most commonly arises from the vestibular component of the vestibulocochlear nerve. In recent surgical studies, the most common origin has been from the inferior vestibular nerve, followed by the superior vestibular nerve.40,41
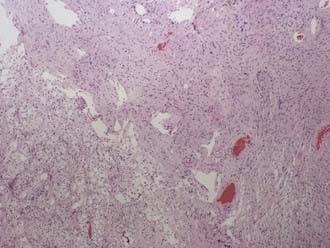
Classically, the microscopic appearance consists of two main histologic patterns: Antoni A and Antoni B (Fig. 133-2). Antoni A areas contain spindle-shaped cells with rod-shaped nuclei and dense reticulin arranged in compact intertwining fascicles. There may be palisading of nuclei forming what is known as a Verocay body. Antoni B areas have stellate or spindled-shaped cells with smaller and more hyperchromatic nuclei, less reticulin, prominent cytoplasmic processes, and a loose myxoid stroma.42 Most acoustic tumors are composed of predominantly Antoni A areas, but a higher component of Antoni B areas may be seen in more cystic tumors. Foamy histiocytes may be observed and are responsible for the bright yellow color associated with the tumor. On occasion, psammoma bodies may be found within an acoustic neuroma because the IAC is lined by dura. Immunohistochemical studies, although not routinely necessary, can be helpful in distinguishing a meningioma from a schwannoma in select cases. Vimentin and EMA are positive in a meningioma, whereas schwannomas express nuclear S-100 and vimentin positivity. Although S-100 can be positive in meningiomas, it is generally focal and cytoplasmic.43
The association of schwannomas with NF2 has led to increased interest in the genetics behind the tumor. Loss of heterozygosity on 22q and deficiency of the protein merlin have been linked to NF2 and have spurred interest in the molecular processes underlying acoustic neuromas. Merlin has been identified as a putative tumor suppressor, and increased understanding of related molecular mechanisms has recently identified potential targets for the development of pharmacotherapy, such as ErbB and Nrg.44
Clinical Findings
Cushing was the first to eloquently describe the progression of symptoms from early sensorineural hearing loss to the later symptoms associated with brainstem compression. Today, as fewer and fewer tumors are escaping detection, patients are less frequently initially seen with overt brainstem compression or hydrocephalus unless the tumor is almost entirely contained within the CPA. Although there is significant variability in the initial clinical findings, the most common initial symptom is asymmetric hearing loss. Asymmetric sensorineural hearing loss is seen in approximately 85% of patients with known acoustic neuromas and is the initial complaint in 65% of patients.45 Tinnitus involving the affected side occurs in a significant majority of patients, and persistent tinnitus should raise concern for an acoustic neuroma. Patients may have vestibular symptoms and complain of progressive imbalance or dizziness. Neurological examination may demonstrate evidence of vestibular dysfunction such as gait ataxia, nystagmus, and a positive Romberg sign. Headaches, facial paresthesia, and facial weakness or fasciculation are currently uncommon initial manifestations of acoustic neuromas.46 Classically, audiologic testing has played an important role in the evaluation of patients with sensorineural hearing loss.
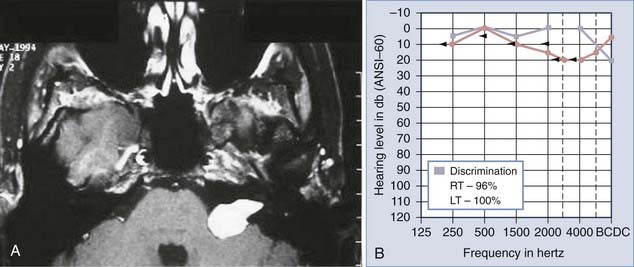
Auditory brainstem response (ABR) testing is the most sensitive audiologic examination for identifying an acoustic neuroma. ABR testing consists of recordings of neural activity within the cochlear nerve and auditory pathways after administration of a stimulus to the patient’s ear. Most large series have reported a sensitivity of 92% to 98% and specificity of 80% to 90% for ABRs.46–48 However, a more recent study found a sensitivity of 71% and specificity of 74% in the evaluation of asymmetric hearing loss. The sensitivity of ABRs was found to be lowest in patients harboring small acoustic neuromas.49 Increased awareness among clinicians has led to earlier diagnosis and changed the goals of surgery from prevention of brainstem compression to the potential for hearing preservation. We have used preoperative ABRs to evaluate the likelihood of hearing preservation. We have never saved hearing in a patient with a latency greater than 2 msec in comparison to the normal ear (Fig. 133-3A and B).
Preoperative Imaging Studies
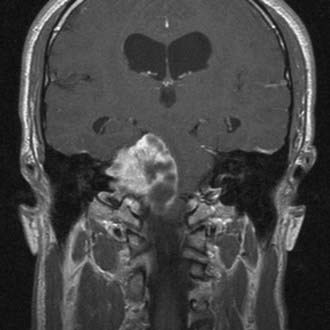
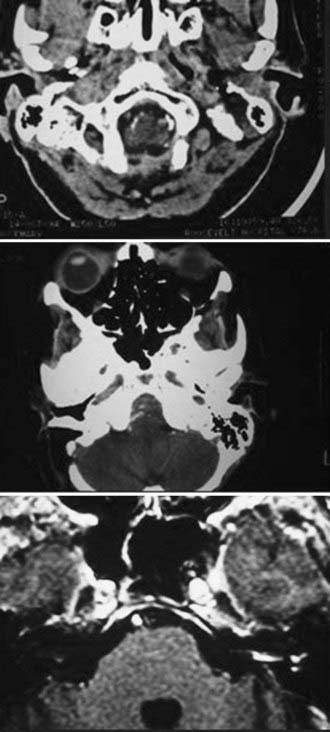
The diagnostic evaluation of patients in whom there is a suspicion of an acoustic neuroma has advanced significantly with the advent of ABR testing, computed tomography (CT), and MRI. MRI and in particular MRI with paramagnetic contrast material have greatly increased the sensitivity of imaging in the detection of acoustic neuromas and in the distinction between other CPA pathology (Fig. 133-4). The sensitivity and specificity have increased so much that the utility of ABRs as a screening measure for asymmetric sensorineural hearing loss has been questioned.49 The use of specific “IAC” protocols to evaluate the CPA and bilateral IACs provides high-resolution, thin-cut sequences in a timely manner. Acoustic neuromas are the most common tumor seen within the CPA and account for 70% to 80% of tumors, followed by meningiomas and then epidermoids.50 Acoustic tumors usually demonstrate isointensity to brain parenchyma on T1-weighted sequences and hyperintensity on T2-weighted sequences.51,52 Acoustic neuromas demonstrate avid contrast enhancement after the intravenous injection of gadolinium. Tumors can have variable enhancement patterns: homogeneous (50% to 60%), heterogeneous (30% to 40%), or cystic (5% to 15%).37,51,52 The MRI appearance of the tumor varies somewhat depending on the size of the tumor and its histologic composition. Homogeneous small tumors are composed predominantly of the Antoni A type, whereas larger, more heterogeneous tumors have mixed Antoni A and Antoni B types or only the Antoni B type.52 In addition to aiding in the diagnosis of an acoustic neuroma, MRI also provides additional anatomic information useful in planning treatment. Information regarding the size, intracanalicular component, and CPA component is provided and can aid in planning treatment. Three-dimensional, fast spin-echo, heavily T2-weighted sequences such as FIESTA (fast imaging employing steady-state acquisition) and CISS (constructive interference in steady state) can also provide additional information regarding other CPA cranial nerves, including the facial nerve (Fig. 133-5). However, imaging never replaces the need for intraoperative observation and neurophysiologic monitoring because the facial nerve is often intimate with the tumor capsule.
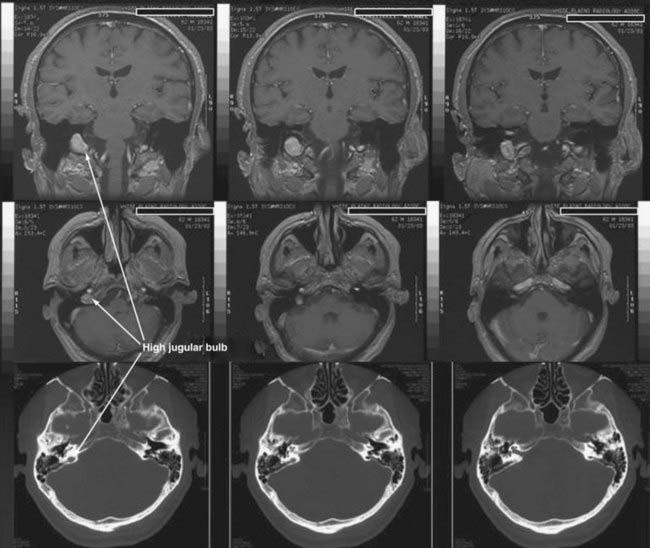
CT performed with bone algorithms can provide details on the bony anatomy not evident on MRI and still plays an important role in the preoperative evaluation of patients and in particular in surgical planning. The presence of a high-riding jugular bulb and its proximity to the IAC is important to be cognizant of when planning a retrosigmoid approach because it can limit lateral exposure of the tumor, particularly when performed with the patient in a supine position (Fig. 133-6). CT also provides useful information about the extent of mastoid and adjacent bone pneumatization, which is important in preventing postoperative cerebrospinal fluid (CSF) leaks (Fig. 133-7).
Preoperative Management
Discussion of all options available to a patient with a newly diagnosed acoustic neuroma is crucial. Patients can ultimately decide to be managed by observation until growth is observed or new clinical findings occur, or they may choose surgery or radiation therapy. Observation is an acceptable option for many patients with small or medium-sized tumors, particularly in elderly patients.53 Patient age, hearing status, and size of the tumor all play an important role in choosing to observe an acoustic tumor rather than intervene. The natural history of acoustic tumors has been described and is represented in Table 133-1. Measurable tumor growth demonstrated on MRI is a good predictor of future tumor growth and should encourage active management.
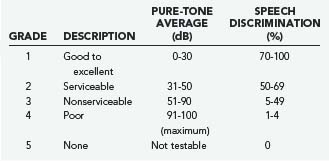
In general, initial follow-up MRI within 1 year is indicated to establish a growth trajectory. Annual scans for 3 to 5 years followed by every 2 years until 10 years and every 5 years thereafter is reasonable, but there is not sufficient evidence to suggest what the standard of care should be. Patients with useful hearing at the time of diagnosis are likely to lose hearing with a policy of observation inasmuch as more than half of patients lose hearing during the observation period.53 The specifics of the case will frequently determine the approach taken, and it is fruitless to generalize. Pure-tone audiometry and speech discrimination testing are usually performed, and hearing can be classified by using the scale proposed by Gardner and Robertson (Table 133-2).54,55 This provides an objective measure that can be followed and is useful in management decisions.
Surgical Approaches
There is much debate in the literature regarding the benefits of the three main approaches to an acoustic neuroma: the retrosigmoid, middle fossa, and translabyrinthine approaches. Although there are potential benefits and limitations with each approach, it is important to not be dogmatic about which surgical approach produces superior outcomes. The best approach in a particular case reflects the goals of the surgery, functional status of the patient, and the experience of the operative team with the proposed surgical approach. A learning curve is present with resection of acoustic neuromas, and the results therefore represent a moving target.56,57 Studies are thus limited by the particular surgeon’s preference and personalized results with each approach.
The translabyrinthine approach is not appropriate when hearing preservation is a goal. Some have suggested that the middle fossa approach is preferable in patients with a small, laterally located intracanalicular tumor and hearing preservation as a goal (see Fig. 133-7).58–60 However, excellent results in terms of hearing preservation can also be achieved via a retrosigmoid approach. Samii and coauthors recently reported a series of 200 patients in whom they achieved 51% functional hearing preservation and excellent or good facial function in 81% with a retrosigmoid approach.61 This represents an improvement on their previously reported 1000 patients, thus demonstrating that progress can be made even in the most experienced hands.62 Evaluation of the volume-outcome relationship at 265 hospitals found significantly improved results at hospitals with higher volumes and with surgeons with higher operative caseloads.63 There is clearly a steep learning curve that must be considered when evaluating the effectiveness of different surgical approaches.56,57,64,65 Therefore, conclusions about the appropriate surgical approach are thus limited by the personal experience of the surgeon and the particular aspects of the case (Table 133-3).
TABLE 133-3 Hannover Tumor Extension Classification System
| CLASS | TUMOR EXTENSION |
|---|---|
| T1 | Intrameatal tumor |
| T2 | Intrameatal and extrameatal tumor |
| T3A | Tumor filling the cerebellopontine cistern |
| T3B | Tumor reaching the brainstem |
| T4A | Tumor compressing the brainstem |
| T4B | Tumor severely displacing the brainstem and compressing the fourth ventricle |
From Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): facial nerve preservation and restitution of function. Neurosurgery. 1997;40:684-695.
Retrosigmoid
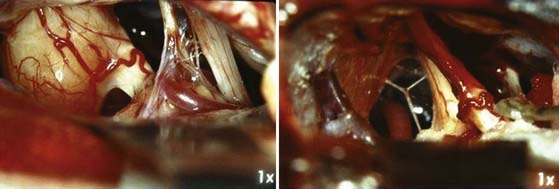
The standard retrosigmoid approach is the most familiar to neurosurgeons and has many advantages. One major advantage is the flexibility of the approach with regard to tumor size and extracanalicular extension. One can address large extrameatal tumors causing compression of the brainstem to small intracanalicular tumors through a similar approach. There is an excellent view of the cranial nerves, and smaller tumors allow the surgeon to attempt hearing preservation (Fig. 133-8).
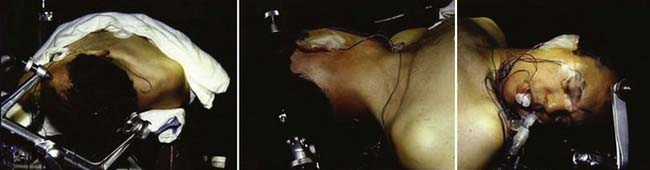
The semisitting, supine, supine-oblique, park bench, and lateral oblique positions have been used for suboccipital removal of acoustic neuromas.66 Most frequently, a supine position with shoulder bolsters is used at our institution (Fig. 133-9). It is important to make certain that the patient is secured to the operative table with multiple belts because there is frequent rotation of the table to improve exposure during surgery. Care should be taken to ensure that the patient has adequate padding at all potential pressure points. Three-point Mayfield skeletal fixation is used. The patient’s head is then turned to the contralateral side. If the extent of lateral rotation of the head is limited secondary to cervical spondylosis, a lateral position can be used. The key to adequate exposure of the posterior fossa and CPA is achieving a balance between the amount of flexion and rotation. With too little rotation, exposure of the CPA will be compromised, whereas too extensive rotation runs the risk of venous occlusion. We routinely give 0.5 to 1.0 g/kg of mannitol, 1.5 g of cefuroxime, and 10 mg of dexamethasone before the incision.
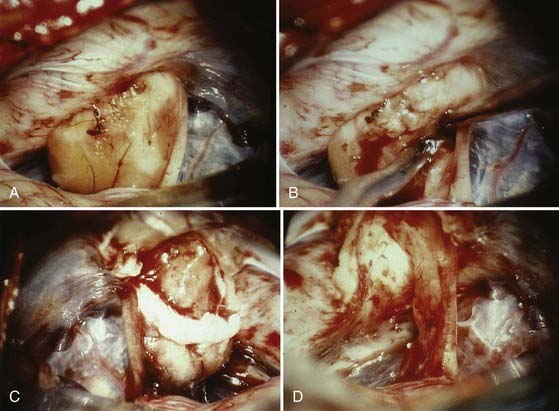
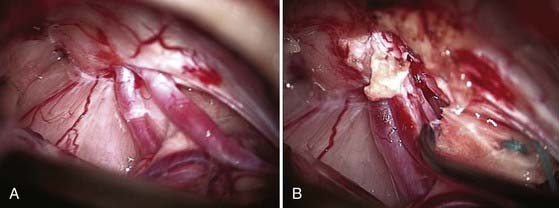
The operative microscope is brought into the field and the remainder of the surgery performed with microsurgical technique. The tumor is identified and the visualized cranial nerves are protected with a cottonoid. The arachnoid is dissected from the posterior aspect of the tumor. The posterior middle third of the tumor is the most commonly used entry zone for internal debulking. The facial nerve is usually found anteriorly in the middle third of the capsule, with the posterior part of the tumor being the least likely location of the nerve.67 A facial nerve stimulator is used to stimulate the dorsum of the tumor and confirm the absence of the facial nerve before initiating internal debulking. Although less common, both the facial and cochlear nerves can be found posterior to the tumor, thus emphasizing the importance of using a nerve stimulator before initiating the debulking (Fig. 133-10). The tumor is removed with ultrasound aspiration, microdissectors, and microcup forceps. Bipolar electrocautery is used sparingly, and we prefer to use Surgicel or Gelfoam for hemostasis to limit the potential for inadvertent cranial nerve injury. Once internal debulking of the tumor is adequate, the tumor capsule is gently moved into the field while maintaining the arachnoid plane (Fig. 133-11A-D). A facial nerve stimulator is again used to verify that there is no involvement of the capsule with the facial nerve. Whenever possible, early identification of the cranial nerves in the CPA extending to the porus acusticus is recommended.
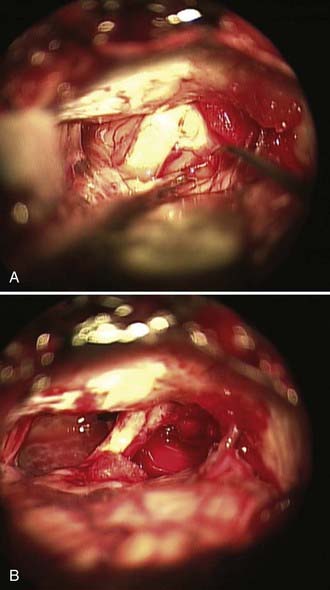
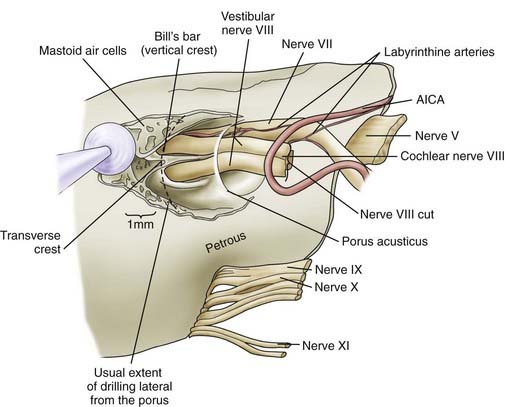
We routinely use a team approach, and our neurotologist generally drills the bone to open the posterior wall of the IAC. We place Gelfoam in the subarachnoid space to decrease the amount of bone dust accumulation during drilling of the IAC and thereby prevent severe postoperative headaches (Fig. 133-12).68 Once the dura on the posterior surface of the petrous ridge is removed, a diamond bur is used to uncover the IAC from its superior to its inferior surface. Particular care is taken to not enter the inner ear because the posterior semicircular canal and vestibule are in close proximity to the drilling. If a semicircular canal is breeched, it should be immediately packed and sealed with bone wax because hearing can still be preserved.69,70 The dura covering the canal is removed to expose the nerves as they enter the canal. The different contents of the IAC are identified, with the facial nerve being superior/anterior and separated from the superior/posterior superior vestibular nerve by Bill’s bar. The cochlear nerve is inferior/anterior and the inferior vestibular is inferior/posterior, with separation from the superior contents of the canal by the transverse crest (Fig. 133-13). The tumor is separated from the cochlear and facial nerves by progressing from either a lateral-to-medial direction or a medial-to-lateral direction, depending on the ease of separation of the planes. Both the facial nerve and cochlear nerve are monitored closely during this step to ensure that physiologic function is maintained during the dissection. The tumor is removed completely, after which the facial nerve is stimulated near the brainstem to assess its integrity.
Once the tumor has been removed, close attention is paid to hemostasis with various hemostatic agents. Any air cells within the canal are identified and carefully sealed with bone wax, muscle, or a combination of the two. This prevents a source of CSF leak via the eustachian tube. Endoscopy at this stage is helpful to ensure that all lateral tumor has been removed and all air cells have been sealed.68 The posterior fossa is irrigated throughout the procedure, particularly during drilling to limit the amount of bone dust and debris. Bone dust can contribute to both postoperative headaches and decreased arachnoid granulation drainage of CSF, which can lead to hydrocephalus. Before closing the dura, the CPA is inspected and irrigated as necessary. The dura is closed in watertight fashion with interrupted suture, and DuraSeal is applied over the suture line. The craniectomy is again inspected for evidence of air cells, and any additional air cells identified are waxed. A titanium mesh cranioplasty with methyl methacrylate is performed, or alternatively in the case of a craniotomy, the bone flap is replaced.71
Middle Cranial Fossa
The middle cranial fossa approach initially introduced by House for acoustic neuroma surgery has been described by many for hearing preservation surgery for small laterally located intracanalicular tumors.58,72–76 Comparison of this approach with the retrosigmoid approach remains a topic of debate, with excellent outcomes achieved with each approach in skilled hands. This approach is not ideally suited for patients with significant extracanalicular extension because of poor visualization of the CPA and brainstem, although extended middle fossa approaches have been used.
A linear vertical incision, curved incision, or a horseshoe incision is made through the skin and should be concealed behind the hairline whenever possible. A periosteal elevator is used to elevate the mucoperiosteal layer. A craniotomy approximately 4 cm in diameter is performed. The bone flap is dissected free from the dura and passed off for storage until the end of the procedure. If the inferior aspect of the craniotomy is not flush with the floor of the middle cranial fossa, a rongeur is used to carry the craniotomy inferiorly until it is flush. The microscope is brought in for elevation of the temporal dura off the floor of the middle cranial fossa. A House-Urban retractor is used for gentle medial retraction of the temporal lobe.77 Dissection proceeds in a posterior-to-anterior direction to avoid inadvertent injury to the greater superficial petrosal nerve, geniculate ganglion, or dehiscent petrous carotid. Anteriorly, the dissection is complete when there is adequate visualization of the foramen spinosum and middle meningeal artery. The posterior petrous ridge is identified.78
The temporal floor is evaluated for landmarks to identify the IAC. One method is to start drilling on the most medial aspect of the straight line parallel to the external auditory canal and perpendicular to the inner table. Another method is to draw a 100-degree angle between the greater superficial petrosal nerve and the arcuate eminence. The apex of the angle is bisected and drilling performed over the medial aspect of the petrous ridge.78,79 Drilling is performed with a diamond bur in a medial-to-lateral direction toward the fundus. Close attention is paid to avoiding injury to the underlying cochlea anteriorly and the labyrinth posteriorly. The geniculate ganglion and labyrinthine segment of the facial nerve are exposed.77 A direct nerve monitor can be applied to the anterior-inferior extradural region for nearly real-time monitoring of cochlear nerve function.
The dura of the IAC is incised along the posterior aspect and reflected anteriorly. The facial nerve is dissected away from the tumor starting at Bill’s bar. The tumor is then debulked internally and removed in a medial-to-lateral direction. The AICA is identified and dissected from the tumor to avoid hemorrhage. This is particularly challenging with larger tumors because they more often have complex involvement with the AICA (Fig. 133-14A and B).67 The cochlear nerve is dissected from the tumor, and the superior vestibular nerve can be sectioned if it is the origin of the tumor. Any change in ABRs should alert the surgeon that there could be too much traction on the cochlear nerve or stretching of the labyrinthine artery.80 Hemostasis is verified before closure, and an abdominal free fat graft is placed over the IAC. The bone around the IAC is inspected for air cells and bone wax applied as necessary. The bone flap is replaced and skin closure completed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree