CHAPTER 289 Acquired Abnormalities of the Craniocervical Junction
The craniocervical junction is the most complex portion of the axial skeleton. The geometry of the articular surfaces provides mobility at the cost of stability. The latter is provided by the ligamentous structures as well as the cervical musculature.1 The synovial lining of the craniocervical joints is affected early in rheumatoid disease and plays a significant role in the subsequent destructive changes that occur in this region.2,3 Other nonrheumatoid entities that affect the craniocervical junction are inflammatory (e.g., ankylosing spondylitis, juvenile rheumatoid arthritis, psoriasis, regional ileitis, Reiter’s syndrome), degenerative (e.g., osteoarthritis, calcium pyrophosphate pseudogout), and infective (e.g., pyogenic, Grisel’s syndrome).4
Normal Anatomy
The complex relationships between the occipital bone and the first and second cervical vertebrae constitute the occipitocervical junction. The foramen magnum is composed of three parts: the squamosal portion dorsally, the basal portion ventrally, and the condylar portions laterally. The occipital condyles are prominences that articulate with the lateral masses of C1. C1 is unique among the vertebrae in that it is ring-shaped, without a vertebral body. Instead, the dens of C2 occupies this position within the ring of C1. The lateral masses are located at the anterolateral portions of the C1 ring. The superior articular processes of C1 join with the occipital condyles, and the inferior articular processes join with C2, allowing a significant degree of flexion and extension movement at the occipitoatlantoaxial complex. Each of these joints, along with the articulation between the dens and the anterior arch of C1, is a true synovial joint.5,6
The complex ligaments of the occipitocervical junction provide some stability to the region while also allowing a significant range of motion. The cruciate ligament is the most important of these structures. This ligament is composed of two portions: the transverse portion, which runs between the lateral aspects of the C1 ring dorsal to the dens, and the cervical portion, which runs from the basion to the C2 vertebral body, also dorsal to the dens. In addition to the cruciate ligament, there is an apical ligament connecting the tip of the dens to the basion, two alar ligaments running from the anterolateral portions of the foramen magnum to the tip of the dens, and the paired accessory atlantoaxial ligaments securing the lateral masses of C1 to the body of C2. In addition to these ligamentous structures, the tectorial membrane provides support to this region. This membrane is the rostral extension of the posterior longitudinal ligament and runs from C3 to the clivus.6
The craniovertebral junction receives its blood supply from the vertebral and occipital arteries. These vessels anastomose to form the apical arcade over the superior aspect of the dens. These feeding vessels, the apical arcade, and branches from the carotid arteries provide most of the blood supply to this region. The lymphatic ducts of this region drain primarily into the retropharyngeal space and subsequently to the deep cervical chain. This pattern of lymphatic drainage explains the development of Grisel’s syndrome, whereby inflammation of the craniocervical synovial joints develops following infection of the nasopharynx or retropharyngeal space.6
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is one of the most common disabling diseases today. The overall prevalence in Europe of proven RA is 0.8% of those older than 15 years of age.7 The prevalence in Sudbury, Massachusetts, is similar, at 0.9%,8 and a national sample of the white population in the United States yielded a prevalence of 1%.9,10 Thus, the number of patients with RA in the United States is about 2 to 2.4 million, and in the United Kingdom it is 0.63 to 0.65 million. Based on the various studies available, a significant number will develop cervical spine involvement requiring attention.
RA of the cervical spine was first described as a clinical entity by Garrod11 in 1890. In that series of 500 patients, 178 had involvement of the cervical spine. According to Conlon and coworkers,12 if lateral cervical radiographs were obtained among the general population, cervical spine involvement compatible with RA would be detected in about 6%. However, the clinical involvement is a fraction of this. Based on their initial studies, Conlon and coworkers believed that involvement of the cervical spine did not cause neurological deficits. Unfortunately, this led to the common but erroneous belief that treatment of rheumatoid involvement of the craniocervical junction should be conservative. However, in 1974, Mathews13 pointed out that progressive atlantoaxial subluxation occurred in up to 25% of RA patients, and rheumatoid cranial settling occurred in 6% to 18% of individuals.
RA is a chronic relapsing inflammatory arthritis that usually affects multiple diarthrodial joints with varying degrees of systemic involvement.14–16 RA occurs worldwide and in all racial groups; it affects females two to four times more frequently than males. It causes substantial morbidity and an attendant economic burden; approximately 50% of patients are unable to work within 10 years of onset, and the lifetime cost of the disease rivals that of coronary artery disease or stroke.16 Joints, articular tissues, serosae, and the eyes are commonly affected, but the spectrum of organ damage may be vast, especially when vasculitis develops during the course of the disease.17
Immunologic Features
Rheumatoid factors are antibodies with specificity for the Fe fragment of immunoglobulin G (IgG).17,18 The latex agglutination test is positive in 80% of patients meeting the American Rheumatism Association criteria (Table 289-1).19 High titers are associated with the presence of subcutaneous nodules, extra-articular manifestations, and vasculitis. A negative rheumatoid factor test by routine laboratory procedures does not exclude the diagnosis of RA because 20% of patients who meet the American Rheumatism Association criteria test negative for RA. These patients may have IgG or IgM rheumatoid factor that is not detectable by routine techniques.20
TABLE 289-1 American Rheumatism Association Criteria for Classic or Definite Rheumatoid Arthritis
Antinuclear antibodies have been found in 14% to 28% of patients with RA. Tests that use monoclonal rheumatoid factors in the CLq binding assay are more frequently positive in patients with RA than all the various assays for the detection of immune complexes.15 Although the assays correlate poorly with indices of disease activity, a positive test is usually associated with an increased incidence of extra-articular manifestations, particularly vasculitis.
More recently, anti–cyclic citrullinated peptide (anti-CCP) antibodies have been identified in patients with RA. These antibodies react to a variety of citrullinated proteins, including filaggrin and keratin. These antibodies have been identified in the serum of patients before the onset of RA symptoms, suggesting that they play a role in the pathogenesis of the disease. Anti-CCP antibodies have a greater specificity for RA than does rheumatoid factor. Taken together, positivity for rheumatoid factor and anti-CCP antibodies is 98% specific for RA.21
The cause of RA is unknown. However, it is postulated that the disease is triggered by an infectious agent. Macrophages present this antigen on their surface, inciting the release of cytokines such as tumor necrosis factor (TNF) and initiating the inflammatory cascade. This macrophage activation is dependent on human leukocyte antigen (HLA) molecules, explaining the predisposition for RA in patients with particular HLA haplotypes (HLA-DR4 and HLA-DR1). In addition to macrophages, T cells (predominantly Th1 cells), B cells, plasma cells, natural killer cells, dendritic cells, and mast cells infiltrate the synovium, transforming it into the rheumatoid pannus. T cells interact with B cells, which subsequently secrete autoantibodies that further promote the inflammatory response through the recruitment and activation of complement and neutrophils. In addition, synovial fibroblasts respond, developing into RA synovial fibroblasts, which play an active role in joint destruction. RA synovial fibroblasts, macrophages, and T cells secrete a number of chemokines, including interleukin-1β, interleukin-17, and TNF, which promote the production of matrix metalloproteinases. Other molecules such as cathepsins, collagenases, and stromelysin are secreted and participate in the destructive processes. In addition, the cytokine milieu results in the activation of osteoclasts.22–24
Rheumatoid pannus forms in the inflamed joints from proliferating fibroblasts and inflammatory cells, leading to granulation tissue.25 The pannus itself produces collagenase and proteolytic enzymes capable of destroying adjacent cartilage, tendons, and bones.26 In addition, some patients develop lymphoid germinal centers within the synovium consisting of follicular aggregates of T cells, B cells, and dendritic cells.21 The end result is loss of cartilage, ligamentous laxity, rupture of tendons, and bone erosion. In general, the joints ultimately undergo severe destruction and become symptomatic within the first year of disease onset.
Clinical manifestations of RA include constitutional symptoms; arthritis involving large, medium, and small joints, and extra-articular manifestations that include subcutaneous nodules, nail bed thrombi, pleurisy, pulmonary fibrosis, pericarditis, nerve entrapment syndromes, scleritis, and vasculitis. The most common vascular complications are peripheral cutaneous ulcers and neuropathy.27–31
The earliest changes in the cervical spine most likely take place at the superficial joints and the lateral margins of the disks. Neurocentral synovitis results in granulation tissue with fibroblasts and capillaries that erode and replace the disk annulus and neighboring disk-bone border. This is not accompanied by active bone formation.32 Therefore, osteophyte formation around disks and apophyseal joints is inconspicuous, enhancing the possibility of dislocation.13,33,34
Fibrosis and ankylosis of apophyseal joints are not uncommon in untreated cases, and there is a natural tendency for the disease to terminate in segmental immobilization, with loss of disk and stepwise subluxation in the subaxial cervical region. The active inflammatory lesions with fibrinoid changes are similar to those in the tendons of the hand and are thus seen in the apical ligaments, the transverse ligament of the craniocervical junction, and biopsy specimens of the interspinous ligaments.35 The transverse ligament becomes insufficient because of inflammatory erosion of the posterior surface of the odontoid process by granulation tissue arising in the synovial joints between the transverse ligament and the posterior surface of the odontoid process. Osteoporosis is a frequent finding in the rheumatoid cervical spine and may contribute to weakening of the bone beneath the ligamentous attachments.35,36
Rheumatoid Involvement of the Spine
The cervical spine is the most common site affected because of the large number of synovial joints. There is a predilection for involvement of the craniocervical junction for this reason. The most common lesion found is atlantoaxial dislocation, followed by cranial settling and then rheumatoid granulation tissue. Subaxial subluxation occurs in 12% to 22% of individuals and is seen predominantly at the C4 and C5 levels.37 Winfield and colleague38 evaluated 100 rheumatoid patients diagnosed within 1 year of the onset of disease. At 5-year follow-up they found that 12 patients had atlantoaxial subluxation of more than 7 mm, and subaxial subluxation had occurred in 20. In 3 individuals, vertical migration of the odontoid process had occurred. Zikou and coworkers39 reported a series of 165 consecutive patients with RA, 66 of whom reported symptoms of cervical spine involvement, including neck pain, neck stiffness, and limited cervical range of motion. Interestingly, 146 of 165 patients had radiographic evidence of rheumatoid disease of the cervical spine, including 80 patients who had no symptoms referable to the cervical spine. The radiographic findings included atlantoaxial subluxation greater than 2.5 mm (20.6%), subaxial subluxation greater than 1 mm (43.6%), and odontoid erosions (2.4%). This same group reported on a series of 51 patients with RA who underwent magnetic resonance imaging (MRI) investigation of the cervical spine. Thirty of these patients had clinical evidence of cervical spine disease, and 40 had other radiographic evidence of cervical spine involvement. On MRI, 88% of the patients demonstrated some degree of pannus formation at the dens, 23.5% had dens erosion, 13.7% had atlantoaxial subluxation, and 10% had subaxial subluxation.40 In longitudinal studies, it appears that atlantoaxial subluxation begins with horizontal subluxation, progresses to a combination of vertical and horizontal subluxation, and ultimately develops into vertical subluxation alone in a subset of patients. It is rare for patients to develop vertical subluxation alone without first passing through a stage of horizontal subluxation. More severe atlantoaxial subluxation was observed in patients with more severe systemic disease.41
Atlantoaxial subluxation is initiated by a loss of tensile strength and stretching of the transverse ligament. Similar changes occur in the synovial joints around the odontoid process as in the joints of the lateral atlantoaxial and occipitoatlantal regions. Thus, there are erosive changes in the adjacent bone, formation of granulation tissue in the synovial joints, loss of bone volume, osteoporosis, angulation of the softened bone, and occasionally fracture. The laxity of the ligaments, together with the bone changes, leads to horizontal or anteroposterior translation as well as rotary luxation of the atlas and the axis vertebrae (Fig. 289-1).42–49
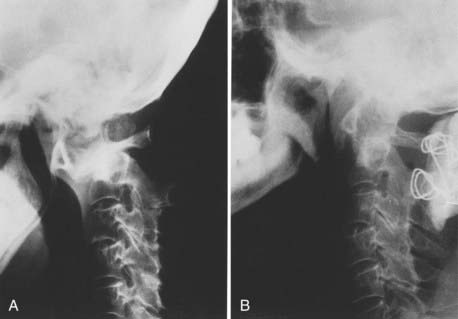
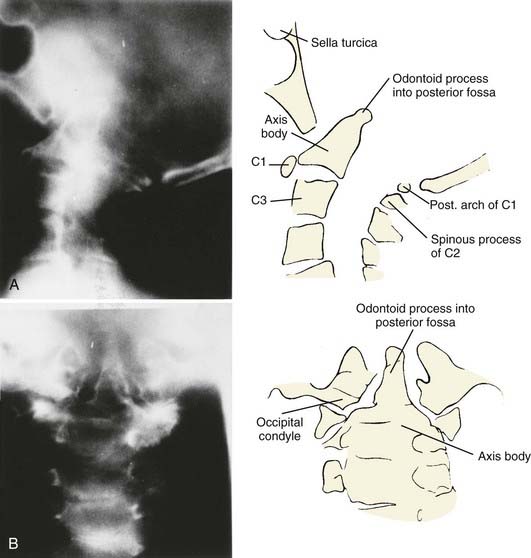
Vertical penetration of the odontoid process into the foramen magnum has been variably termed rheumatoid basilar invagination, odontoid vertical migration, rheumatoid translation, and cranial settling.9,13 It is caused by the loss of bone and the lateral mass of the atlas vertebra, with rostral migration of the axis. The lateral atlantal masses may fracture in severe cases with lateral displacement.25 The odontoid then penetrates further through the foramen magnum. The destructive changes may be severe enough that the occipital condyles rest on the lateral masses of the axis vertebrae, separating and displacing the anterior and posterior arches of the atlas and their respective directions (Fig. 289-2).50 The anterior arch of the atlas settles downward, telescoping onto the axis body, and there is an upward invagination of the odontoid process. This is not basilar invagination or basilar impression but cranial settling.9,25,28,51–53 Casey and colleagues54 reported on a prospective review of 256 patients with RA. Of the 186 patients with cervical myelopathy, 62% had cranial settling (vertical translocation). The patients with cranial settling had more severe neurological dysfunction than those with horizontal subluxation. In addition, patients with cranial settling experienced pseudostabilization, with a decrease in the atlantodental interval and a resulting decrease in the size of the pannus.
Excessive proliferation of granulation tissue may lead to complete destruction of the odontoid process, with the granulation mass and pannus emanating out of the rest of the synovial joints.31 This causes ventral and lateral cervicomedullary compression with gross dislocation in all directions (Fig. 289-3). At times the bony spicule of the odontoid process may be left as a ghost and can penetrate the tectorial membrane, subsequently embed in the ventral aspect of the pons and the medulla, and attach itself to the vertebrobasilar arterial tree. Occasionally, rheumatoid dural nodules and pachymeningitis occur secondary to the rheumatoid process. The rheumatoid pannus may be associated with granulation tissue and become tough and fibrotic.31 This does not usually recede with immobilization. In contrast, active pannus that resembles the active disease in other joints recedes with cervical immobilization.53,55–57
Subluxation below the second cervical vertebra occurs in 17% to 29% of patients with RA, most commonly at the C3-4 and C4-5 levels. Serial cervical subluxations producing a staircase appearance are common.12,58
The natural history of rheumatoid disease of the cervical spine is grim. Fujiwara and associates41 reported on a series of 173 patients with RA followed from 1992 to 1997. Initially, 29% of the patients had atlantoaxial subluxation. Over the 5-year period, 63% of these patients experienced progression of their atlantoaxial disease. In addition, 39% of the patients who initially had no atlantoaxial subluxation developed upper cervical spine disease over the course of the study. At the beginning of the study period, no patients had neurological deficits; by the end of the study, 10 patients were myelopathic. Pellicci and coworkers59 studied RA patients over a 5-year period and found a mortality rate of 17%, as opposed to a 9% mortality rate for the same age group without rheumatoid disease. Subluxation worsened in 80%, and new subluxation occurred in 27%. Mikulowski and associates60 reported on a postmortem study of 104 patients with RA and found the cause of death in 11 patients to be atlantoaxial dislocation with cervicomedullary compression. This had not been suspected antemortem. Two patients had myelomalacia, and three others had cervical vertebral artery vascular complications related to dislocation. In 1981 Marks and Sharp33 reported on 31 patients with RA and upper cervical spine involvement with dislocation. Fifteen deaths occurred within 6 months of presentation. All those who were untreated died, as did 50% of those treated with a soft cervical collar alone. Only fusion provided a reasonable chance of survival. Delamarter and Bohlman3 performed a postmortem analysis of patients with paralysis secondary to RA of the cervical spine and found cervical cord compression to be the main cause of death in 10. Thus, once cervical myelopathy sets in, the natural history is grave unless surgical intervention takes place.31,61–63
Autopsy analyses of spinal cords from rheumatoid patients suffering from paralysis secondary to atlantoaxial dislocation or cranial settling have shown abnormal histology within the spinal cord at the site of compression.3 Common to all patients were gliosis and axonal degeneration, with nuclear damage within the ganglion cells frequently appearing in different stages of progression. No parenchymal hemorrhage was seen. Delamarter and Bohlman3 identified three histologic types of spinal cord compression. In the first type, four patients with severe, chronic mechanical cord compression exhibited distortion with flattening and destruction of the cord and secondary wallerian degeneration of the ascending and descending tracts, without anoxic or ischemic neural changes, suggesting that the damage was due to chronic mechanical compression. In the intermediate stage of compression (type II), there was vascular compression showing ischemic damage to the cord, with necrosis of the lateral columns and the ischemic watershed regions supplied by the anterior and posterior spinal arteries. In the patients with mild mechanical compression (type III), there was only focal gliosis at the site of compression, without ascending or descending tract injury. Nakano and coworkers45 analyzed two autopsy cases and found that maximal changes occurred in the central gray matter and adjacent posterior and lateral columns. This was thought to be due to direct intermittent compression and narrowing of the transverse branches of the anterior spinal artery. This was previously observed by Bland2 and confirmed more recently by Casey and colleagues.62 Thus, the available space for the spinal cord at the craniocervical junction is of great prognostic significance.4,62,63
In addition to the cervical involvement described earlier, patients with RA are at higher risk for the development of compression fractures in other areas of the spine. Orstavik and associates64 showed that patients with RA had a significantly higher risk of multiple thoracic and lumbar compression fractures than matched controls (odds ratio 2.60). In this study, presence of RA, bone mineral density, and corticosteroid use were independent predictors of vertebral deformities. In addition, there are reports of spontaneous (nontraumatic) odontoid fractures in patients with RA. Although rare, this condition should be considered in RA patients with neck pain whose work-up is otherwise unrevealing.65,66
Extra-articular Manifestations
Extra-articular manifestations are myriad in patients with severe RA and high titers of rheumatoid factor. Pericarditis, myocarditis, and coronary vasculitis are not infrequent.20,29,30,67 RA can present in the lungs as pleural effusions and pleuritis, intraparenchymal rheumatoid nodules, and Caplan’s syndrome (rheumatoid nodules with pneumoconiosis, diffuse pulmonary fibrosis, bronchiolitis, and pulmonary hypertension out of proportion to coexisting lung disease).18,29,68–70 Pleuritis is the most common thoracic manifestation (5% to 15%), and rheumatoid effusions occur in 50% of patients in the first 5 years after the onset of arthritis.71 Pulmonary fibrosis was seen in 10 of 18 patients with RA who underwent thin-section computed tomography (CT) scanning of the chest, but the plain radiographs were reported as normal.72 Thus, it is important that pulmonary function tests be performed in RA patients before any operative procedure and general anesthesia.
Medical Treatment
Current recommendations for the medical management of RA include the use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and disease-modifying antirheumatic drugs (DMARDs). NSAIDs and cyclooxygenase-2 (COX-2) inhibitors provide symptomatic relief but do not alter the course of the disease. A substantial number of patients are placed on corticosteroids, although the use of this class of medications is limited by side effects. The current mainstay of RA management is DMARDs. Synthetic DMARDs include methotrexate, leflunomide, sulfasalazine, minocycline, intramuscular gold, penicillamine, chloroquine, and hydroxychloroquine. Biologic DMARDs are newer agents that target the immune response. Currently, there are four such medications, three of which inhibit TNF-α (infliximab, etanercept, adalimumab) and one that inhibits interleukin-1 (anakinra). Previously, single DMARD therapy was preferred, but more recent studies have shown a substantial advantage to using combination DMARD therapy.73 A recent study showed that combination DMARD therapy reduces the incidence of atlantoaxial subluxation in patients with RA compared with DMARD monotherapy.74
Clinical Presentation
Atlantoaxial subluxation may present within 2 years of the disease, but it is unusual for myelopathy to develop early.50,59,75 The most frequent symptom of rheumatoid abnormalities of the craniocervical junction is occipital headaches.31 This is typically described as occipital pain radiating to the skull vertex and aggravated by an upright posture. It was present in 60% of patients with atlantoaxial subluxation and 90% of individuals with cranial settling. Between 1977 and 1994, 780 symptomatic patients with RA affecting the craniocervical region were evaluated by Menezes.31 Three main categories of abnormalities were recognized from a radiographic standpoint: atlantoaxial instability, cranial settling, and primary granulation tissue masses.
Cervical myelopathy is insidious, and disability may be mistaken for progression of rheumatoid disease, rheumatoid joint dysfunction, hypothyroidism, or poor nutritional habits.13,25,61,76,77 The clearest indication of cervical myelopathy is progressive physical disability and the inability to carry out daily tasks.45,61 It is difficult to detect abnormal neurological signs of myelopathy in rheumatoid patients with deforming painful arthritis and associated neuropathy. The peripheral neuropathy itself can cause areflexia. Thus, the presence of normal reflexes in a patient with advanced RA should raise the suspicion of myelopathy. We have found neurophysiologic testing with somatosensory evoked potentials to be of no use in this situation, despite some reports to the contrary.9
Limb paresthesia, numbness, weakness, and sphincter disturbance may herald myelopathy. Dizziness, vertigo, and syncope may be associated with vertebrobasilar ischemia. Nystagmus may be evident in this situation. Transient blackout spells were reported by 55% of individuals with cranial settling.25 Gradual, progressive difficulty with ambulation was a chief complaint in 76% of individuals with cervical myelopathy. In 15% of individuals, an acute onset of quadriparesis was seen.31
Abnormal neurological signs in Menezes’31 series included brainstem evidence of internuclear ophthalmoplegia, facial diplegia, nystagmus, spastic quadriparesis, and sleep apnea, especially in patients with cranial settling. These signs, either singly or in combination, were present in 20% of individuals. An additional 20% had loss of pain and touch sensation in the distribution of the trigeminal nerve, and each patient had invagination of the odontoid process in excess of 10 mm above the foramen magnum. The cranial nerves most affected by cranial settling were the glossopharyngeal, vagus, and hypoglossal. With cranial settling, 20% of individuals had evidence of dysfunction of one or more cranial nerves.78 Nine individuals with cranial settling had undergone previous tracheostomy for the diagnosis of rheumatoid pharyngeal dysfunction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree