Acute internal carotid artery (ICA) thrombosis originally diagnosed by cervical duplex ultrasound (A, C, D) and subsequently confirmed by digital subtraction angiography (B). Note the presence of isoechoic material in the ICA lumen both in B-mode (A, C) and color-mode (D) display. Emergent carotid thrombectomy resulted in removal of fresh thrombus (E, F).

Internal carotid artery (ICA) and aortic arch dissection diagnosed using cervical duplex ultrasound that displays the presence of intimal flap causing double-lumen appearance in the ICA (A; B-mode, transverse view) and common carotid artery (B; B-mode, longitudinal view). Takayasu arteritis diagnosed using cervical duplex ultrasound that displays concentric intima–media thickening (C) coupled with elevation of blood flow velocities (D) in the common carotid artery.
| 1. Information about plaque composition and surface (lipid, hemorrhage, fibrous content, ulceration, superimposed thrombus) |
| 2. Diagnosis of degree of carotid artery stenosis (<50%, 50–69%, >70%, near occlusion, occlusion) |
| 3. Diagnosis of degree of vertebral artery stenosis (<50%, >50%, occlusion) |
| 4. Detection of intraluminal thrombus in cervical vessels |
| 5. Diagnosis of subclavial steal syndrome |
| 6. Diagnosis/suspicion of uncommon causes of stroke (cervical artery dissection, fibromuscular dysplasia, aortic arch dissection) |
| 7. Complementary information in the diagnosis of temporal arteritis |
| 8. Indirect information for distal intracranial vessel occlusion |
| 9. Indirect information for heart rate and heart valves |
| 10. Diagnosis of other conditions not related to acute stroke (e.g., carotid body tumor) |
Ultrasound assessment of intracranial arteries
Intracranial cerebral vasculature in patients with AIS can be assessed by performing TCD ultrasound or TCCS to provide real-time flow findings that are complementary to information provided by computed tomography angiography (CTA). Complete or partial TCD examination evaluates up to 16 proximal intracranial arterial segments with the goal of detecting normal, stenosed or occluded intracranial vessels [2,3]. Additionally, TCD is the most convenient method to detect collateral flow and the hemodynamic significance of extracranial or intracranial steno-occlusive lesions, monitor recanalization during thrombolytic therapy in real time, determine the stroke pathogenic mechanism and select the next and most appropriate step in patient management [4,5,6,7] (Table 12.2).
| 1. Bedside confirmation of vascular origin of the presenting symptoms and determination of underlying stroke mechanism |
| 2. Fast detection and localization of occlusion/stenosis |
| 3. Mapping of the collateral circulation |
| 4. Detection of cerebral embolism in real time and quantification of right-to-left shunt |
| 5. Real-time monitoring of recanalization in patients treated with systemic thrombolysis |
| 6. Selection of patients for rescue reperfusion procedures |
| 7. Monitoring of rescue reperfusion procedures (detection of reocclusion, air embolism, hyperperfusion syndrome) |
| 8. Detection of supratentorial intracerebral hemorrhage at the bedside following acute reperfusion therapies (application of TCCS) |
| 9. Potential augmentation of clot lysis and clinical recovery |
Rapid detection of intracranial arterial steno-occlusive disease
A fast-track insonation protocol has been developed for rapid TCD and CDU performance and interpretation, in parallel with the neurologic examination and other necessary testing, in the emergency setting of AIS [8]. The choice of fast-track insonation steps is determined by the clinical localization of the ischemic arterial territory (Table 12.3).
| A. Clinical diagnosis of cerebral ischemia in the anterior circulation | B. Clinical diagnosis of cerebral ischemia in the posterior circulation |
|---|---|
| Step 1: transcranial Doppler | Step 1: transcranial Doppler |
| 1. If time permits, begin insonation on the nonaffected side to establish the temporal window, normal MCA waveform (M1 depth 45–65, M2 30–45 mm) and velocity for comparison to the affected side. 2. If short on time, start on the affected side: first assess MCA at 50 mm. If no signals detected, increase the depth to 62 mm. If an antegrade flow signal is found, reduce the depth to trace the MCA stem or identify the worst residual flow signal. Search for possible flow diversion to the ACA, PCA or M2 MCA. Evaluate and compare waveform shapes and systolic flow acceleration. 3. Continue on the affected side (transorbital window). Check flow direction and pulsatility in the OA at depths of 40–50 mm, followed by ICA siphon at depths of 55–65 mm. 4. If time permits or in patients with pure motor or sensory deficits, evaluate BA (depth 80–100+ mm) and terminal VA (40–80 mm). | 1. Start suboccipital insonation at 75 mm (VA junction) and identify BA flow at 80–100+ mm. 2. If abnormal signals present at 75–100 mm, find the terminal VA (40–80 mm) on the nonaffected side for comparison and evaluate the terminal VA on the affected side at similar depths. 3. Continue with transtemporal examination to identify PCA (55–75 mm) and possible collateral flow through the posterior communicating artery (check both sides). 4. If time permits, evaluate both MCAs and ACAs (60–75 mm) for possible compensatory velocity increase as an indirect sign of basilar artery obstruction. |
| Step 2: carotid/vertebral duplex | Step 2: vertebral/carotid duplex |
| 1. Start on the affected side in transverse B-mode planes followed by color or power-mode sweep from proximal to distal carotid segments. Identify CCA and its bifurcation on B-mode and flow-carrying lumens. 2. Document if ICA (or CCA) has a lesion on B-mode and corresponding disturbances on flow images. In patients with concomitant chest pain, evaluate CCA as close to the origin as possible. 3. Perform angle-corrected spectral velocity measurements in the mid-to-distal CCA, ICA and ECA. 4. If time permits or in patients with pure motor or sensory deficits, examine cervical portion of the vertebral arteries (longitudinal B-mode, color or power mode, spectral Doppler) on the affected side. 5. If time permits, perform transverse and longitudinal scanning of the arteries on the nonaffected side. | 1. Start on the affected side by locating CCA using longitudinal B-mode plane and turn transducer downward to visualize shadows from transverse processes of mid-cervical vertebrae. 2. Apply color or power modes and spectral Doppler to identify flow in intra-transverse VA segments. 3. Follow VA course to its origin and obtain Doppler spectra. Perform similar examination on the other side. 4. If time permits, perform bilateral duplex examination of the CCA, ICA and ECA as described above. |
For example, if middle cerebral artery (MCA) symptoms are present, insonation begins with locating the MCA on the nonaffected side to establish the presence of a temporal window, normal MCA waveform and velocity range. If time permits, insonation of the circle of Willis on the nonaffected side should also provide the depth and velocity range for the M1 and M2 segments of the MCA and the internal carotid artery (ICA) bifurcation. Next, the MCA on the affected side is located with insonation starting at the mid-to-proximal M1-MCA depth range (usually 50–60 mm). The waveform and systolic flow acceleration are then compared to the nonaffected side. If a normal MCA flow is found, the distal M1-MCA segments are insonated (range 40–50 mm), followed by the proximal MCA and ICA bifurcation (range 60–70 mm). If a “blunted” waveform or no signals are found at 50–60 mm, the proximal MCA and ICA bifurcation are insonated before the distal segments. The absence of MCA flow signals can be confirmed by insonation across the midline from the contralateral window (depths 80–100 mm), when feasible. The ophthalmic artery (OA) flow direction and pulsatility is determined next on the affected side (depths 50–60 mm) followed by the ICA siphon assessment via the transorbital window (depths 60–65 mm). The basilar artery (BA) is insonated next to determine if compensatory flow velocity increase or a stenosis is present. Insonation of the vertebral arteries (VAs), OA, and ICA on the nonaffected side, as well as posterior cerebral arteries (PCAs), is performed whenever time permits [9].
Even in TCCS, where the main intracranial arteries including the circle of Willis are easily depictable through color duplex, the Doppler spectral analysis remains the mainstay of diagnosis.
Most studies can be accomplished within minutes and do not delay treatment, if performed by experienced sonographers at bedside. Moreover, TCD and TCCS can be used as a screening tool without CTA for rapid identification of relevant stenosis (Figure 12.3) or occlusion when performed and interpreted by an experienced neurosonologist, since bedside TCD examination performed in the emergency room was found to have a satisfactory agreement with urgent brain CTA in the evaluation of patients with acute cerebral ischemia [10,11,12].
Determination of residual flow at thrombus-blood interface
The TIBI flow grading system was developed to evaluate residual flow noninvasively and to monitor thrombus dissolution in real time (Figure 12.4) [13]. Analogous to the Thrombolysis in Myocardial Infarction (TIMI) flow grades, TIBI waveforms were developed for TCD to predict intracranial vessel patency, and this flow grading system was recently validated against invasive angiography with simultaneous TCD monitoring and intracranial catheter contrast injections [14].
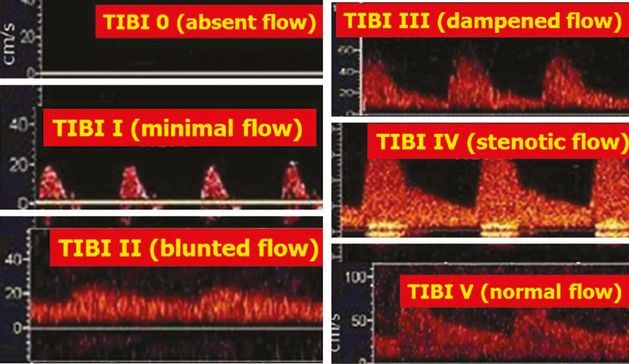
Single-channel TCD display depicting Thrombolysis in Brain Ischemia (TIBI) flow-grading system: TIBI 0: absent flow; TIBI I: minimal flow; TIBI II: blunted flow; TIBI III: dampened flow; TIBI IV: stenotic flow; TIBI V: normal flow.
The TIBI residual flow classification consists of six grades (TIBI: 0–5), which correspond to blood flow that is outlined as follows (Figure 12.4):
Absent (TIBI 0): no flow signals, or lack of regular pulsatile flow signals despite varying degrees of background noise.
Minimal (TIBI I): systolic spikes of variable velocity and duration; absent diastolic flow during all cardiac cycles; reverberating flow is a type of minimal flow.
Blunted (TIBI II): flattened or delayed systolic flow acceleration compared with control side; positive end-diastolic velocity (EDV); pulsatility index (PI) <1.2.
Dampened (TIBI III): normal systolic flow acceleration; positive EDV; ≥30% decrease in mean flow volume (MFV) compared with control side.
Stenotic (TIBI IV): MFV ≥80 cm/s and velocity difference >30% compared with control side; if velocity difference is <30%, look for additional signs of stenosis (i.e., turbulence, spectral narrowing); affected and comparison sides have MFVs <80 cm/s due to low EDVs; MFV >30% compared with control side; signs of turbulence are present during Doppler spectra investigation.
Normal (TIBI V): <30% MFV difference compared with control side; similar waveform shapes compared with control side.
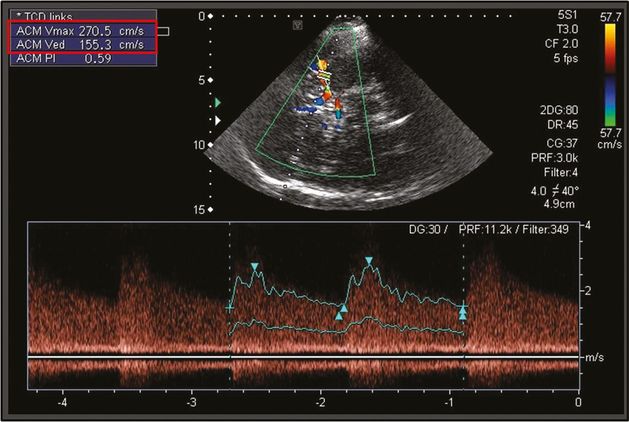
TIBI flow grades I–III correspond to acute proximal intracranial artery occlusion (Figure 12.5), while a TIBI flow grade of IV is indicative of proximal intracranial artery hemodynamically significant (>50%) stenosis (Figure 12.6).
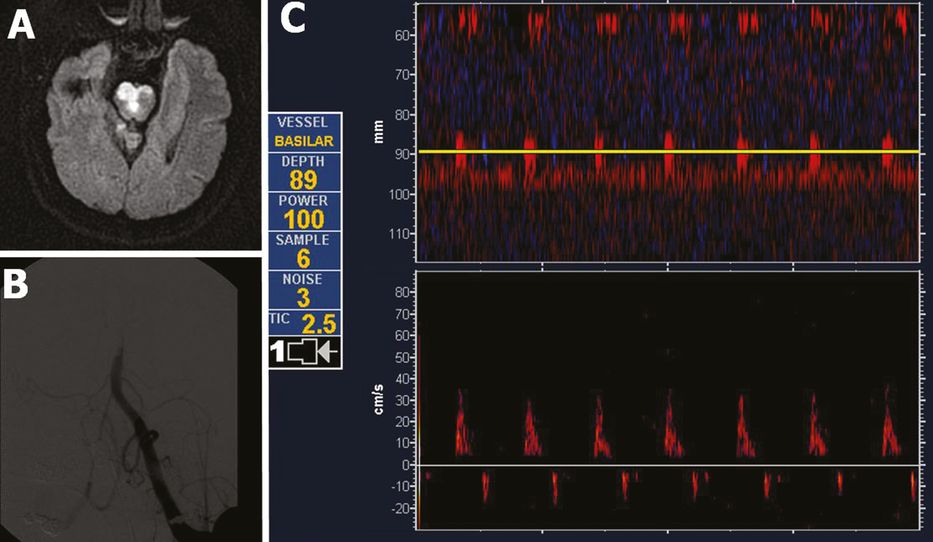
Acute pontine infarction on diffusion-weighted imaging (A) due to middle basilar artery (BA) occlusion diagnosed by power motion-mode display (PMD) transcranial Doppler (C). The patient underwent emergent endovascular reperfusion procedures. The initial digital subtraction angiography diagnostic run is displayed in (B). Note the reverberating high-resistance flow in middle BA both on PMD and spectral displays (C) indicating acute arterial occlusion (residual TIBI flow grade of I: minimal flow).
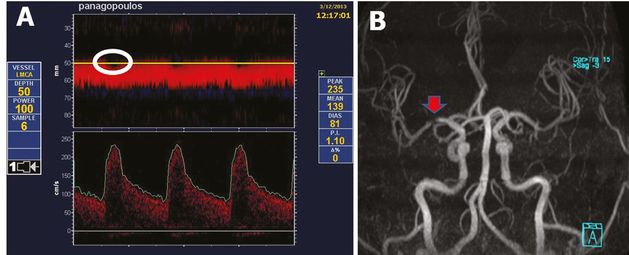
Proximal middle cerebral artery (MCA) stenosis (residual TIBI flow grade of IV: stenotic flow) diagnosed by power motion-mode display (PMD) transcranial Doppler (A) and subsequently confirmed by magnetic resonance angiography (B). Note the systolic flow gaps (white circle) on PMD display (A) indicating turbulent bidirectional flow.
Apart from the likelihood of recanalization, TIBI flow grades were found to correlate strongly with stroke severity, mortality and clinical improvement. TIBI waveforms also reflect an overall resistance to flow, through the changes in velocities during each cardiac cycle, and thus can also be predictive of tissue reperfusion. Recently, this important difference between a proximal recanalization and tissue reperfusion became a focus of scrutiny in acute interventional trials for stroke [15].
Diagnosis of a lesion amenable to intervention
An acute arterial occlusion differs from chronic as it is often partial, incomplete and exhibits dynamic processes (partial obstruction to flow, thrombus propagation, reocclusion and sometimes spontaneous recanalization). TCD can rapidly identify patients with these lesions, regardless of baseline stroke severity, and detect not only the flow-limiting lesion, but also qualify the residual flow around a thrombus, the collateralization, the ongoing embolization and the failure of the vasomotor reserve [16–21].
The noninvasive vascular ultrasound evaluation (NVUE) is a protocol of emergent TCD coupled with CDU in patients with AIS that has a high yield and accuracy in diagnosing lesions amenable to interventional treatment (LAITs), both eligible and ineligible for thrombolysis. A LAIT is defined as an occlusion or near-occlusion, or at least 50% stenosis in an artery supplying brain areas affected by ischemia. Compared with digital subtraction angiography (DSA), NVUE screening criteria (Table 12.4) predicted the presence of LAIT with >90% sensitivity and specificity, despite a 10% rate of no temporal windows.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree