Fig. 3.1
Choroid plexus and the formation of CSF. Fluid and solutes pass freely from the blood through the fenestrated epithelium of choroid plexus capillaries into the stroma (red arrows). CSF is produced by filtration through the choroid plexus epithelial cells
The anatomical substrate of the blood-CSF barrier is the choroid plexus epithelium, which is derived from the ependymal lining of the ventricles (Johanson et al. 2008). Choroid plexuses are formed by the invagination of leptomeninges into the ventricular cavities and by modification of the ventricular ependymal lining into choroid plexus epithelium. Histological and ultrastructural techniques have shown that the choroid plexus epithelium is composed of cuboidal cells. These are coated by basement membrane on their basal surface, which abuts onto the stroma, and by microvilli on the ventricular surface (Wolburg and Paulus 2010). Tight junctions bind the apical portions of these epithelial cells together. CSF is formed by the net transport of water, sodium chloride, potassium and bicarbonate ions from the choroid plexus stroma, through the epithelial cells, into the ventricles (Fig. 3.1) (Johanson et al. 2008). This process involves the enzymes carbonic anhydrase C, sodium and potassium ATPases, and aquaporin -1 (AQP1), which reside in the choroid plexus epithelial cells (Johanson et al. 2008; Wolburg and Paulus 2010; Yool 2007). Acetazolamide reduces CSF production by inhibiting carbonic anhydrase C and by reducing the amount of AQP1 through an alteration in protein transcription (Ameli et al. 2012). CSF in humans is produced at a rate of 0.3–0.6 mL/ min, or 500–600 mL/day. In those smaller mammals that have been studied, CSF is replaced approximately four times per day (Johanson et al. 2008). A small proportion of CSF appears to be derived, additionally, from brain interstitial fluid (Johanson et al. 2008).
3.3 Circulation and Drainage of the CSF
CSF leaves the ventricular system via the foramina of Luschka and Magendie and flows into the basal cisterns and the cisterna magna. It circulates through the ventricles and the basal cisterns and across the foramen magnum in a pulsatile manner (see Sect. 3.5.2), the pulses being derived from the vascular system (Weller 1995).
There are two major routes of drainage of CSF from the subarachnoid channels (Johnston et al. 2004): (a) alongside cranial and spinal nerve roots, particularly the olfactory nerves as they pass through the cribriform plate of the ethmoid bone, and (b) directly into the blood via arachnoid granulations and villi associated with major cranial venous sinuses.
3.3.1 The Ventricular System and Ependyma
The cerebral ventricular system is lined by ependyma, which develops during foetal life. In the postnatal brain and in the adult brain, ependyma consists of a single layer of ciliated cuboidal epithelial cells (Del Bigio 1995), but in the adult human brain, there are frequently areas of the ventricular walls that are devoid of ependyma, leaving the subependymal glia exposed to ventricular CSF. Ependymal cells are joined by gap junctions and lack the tight barrier function of choroid plexus epithelium so that, even in brains with intact ependyma, tracers injected into the ventricles pass freely into the periventricular tissue, particularly into the white matter (Abbott 2004). The central canal of the spinal cord is well defined in the foetus and is also lined by ependyma. However, in the adult human spinal cord, the central canal is usually very small or obliterated and marked only by a small, closely packed nest of ependyma cells.
3.3.2 Leptomeninges and the Subarachnoid Space
The human brain and spinal cord are encased in layers of meninges. On the outer surface, and abutting the bones of the skull and spine, is the tough collagenous dura mater, the outer layer of which forms the inner periosteum of the skull. Within the dura, the leptomeninges consist of two major layers; the outer is the arachnoid mater and is applied to the inner aspect of the dura mater. Separated from the arachnoid by the subarachnoid space is the pia mater. The arachnoid and pia are connected by many sheetlike trabeculae of arachnoid-coated collagen that traverse the subarachnoid space and suspend the leptomeningeal arteries and veins within the CSF (Fig. 3.2) (Weller 2005). As arteries penetrate the surface of the cortex, the arachnoid coating is reflected onto the surface of the brain as the pia mater and a single layer of pia mater also accompanies the artery into the surface of the brain (Fig. 3.2) (Zhang et al. 1990). Scanning electron microscope studies have also shown that the pia mater on the brain and spinal cord is reflected onto blood vessels in the subarachnoid space and thus separates CSF in the subarachnoid space from the brain and spinal cord (Figs. 3.2 and 3.3) (Hutchings and Weller 1986; Nicholas and Weller 1988; Weller 2005). The pia mater is usually only one cell thick and contains intercellular junctions, but it is uncertain how impermeable the pia mater is to the passage of water and macromolecules. Larger particles, such as erythrocytes in subarachnoid haemorrhage, do not penetrate the intact pia mater although inflammatory cells can migrate through this thin cell layer (Hutchings and Weller 1986). Underlying the pia mater, there are bundles of collagen that surround arteries and veins in the subpial space (Alcolado et al. 1988).
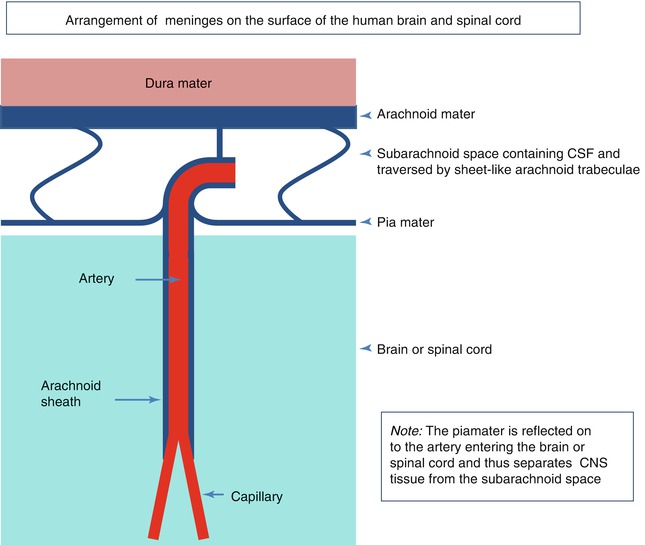
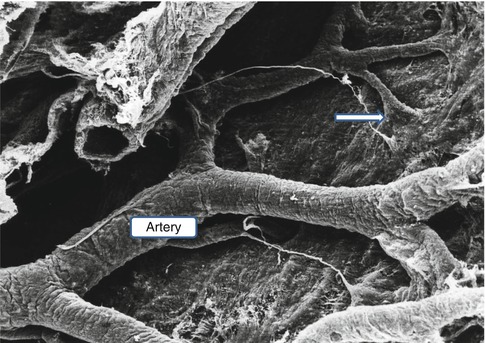

Fig. 3.2
A diagram summarising the arrangement of meninges on the surface of the human brain and spinal cord. Arachnoid mater is closely applied to the dura mater; sheetlike trabeculae cross the subarachnoid space to link arachnoid to the pia mater on the surface of the brain or spinal cord. Pia mater is reflected from the surface of the brain onto the surface of arteries and veins in the subarachnoid space, thus separating CSF in the subarachnoid space from the brain and spinal cord. A thin layer of pia mater extends alongside arteries as they penetrate the brain or spinal cord

Fig. 3.3
Scanning electron micrograph of the surface of the brain as viewed from the subarachnoid space. A leptomeningeal artery spreads its branches over the surface of the pia mater, and before the branches penetrate the brain, the pia mater is reflected on to the surface of the artery (arrow) (Reproduced with permission from Hutchings and Weller (1986)). ×75
The layout of leptomeninges coating the spinal cord differs somewhat from the arrangement of the leptomeninges surrounding the cerebral hemispheres and the brain stem. Arachnoid mater coating the spinal cord is composed of several layers (Nicholas and Weller 1988) (Fig. 3.4). The outer arachnoid is firmly applied to the inner surface of the dura mater. A series of intermediate layers of arachnoid mater are attached to this parietal layer, and they either spread out over the dorsal and ventral aspects of the spinal cord or form the dorsal, dorsolateral and anterior ligaments. Dentate ligaments on the lateral aspect of the spinal cord (Fig. 3.4) are formed of collagenous sheets that connect the dura to the substantial layer of subpial collagen that surrounds the spinal cord. The surface of the dentate ligaments is coated by arachnoid mater (Nicholas and Weller 1988). Functionally, the intermediate layers of arachnoid around the spinal cord may act as baffles, modifying the propagation of pressure waves within the CSF passing up and down the spinal subarachnoid channels.
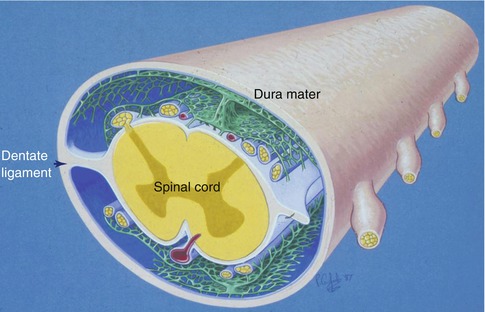

Fig. 3.4
Diagram showing the arrangements of the leptomeninges surrounding the spinal cord. An outer layer of arachnoid mater (blue) is applied to the inner aspect of the dura mater. Highly perforated intermediate layers of arachnoid (green) arise from the outer arachnoid to coat the dorsal and ventral surfaces of the spinal cord. There is a prominent dorsal ligament and less robust dorsolateral ligaments are also present. The dentate ligaments connect the layer of subpial collagen (white) with the collagenous dura (Reproduced with permission from Nicholas and Weller (1988))
3.3.3 Lymphatic Drainage of the Cerebrospinal Fluid
In smaller mammals such as rats, mice and rabbits and even in larger mammals such as sheep, the major pathways for drainage of CSF appear to be alongside cranial and spinal nerve roots to regional lymph nodes. This lymphatic drainage of cranial CSF is via well-defined channels that run alongside branches of the olfactory nerves , as they pass through the cribriform plate of the ethmoid bone to nasal lymphatics (Fig. 3.5) (Johnston et al. 2004; Kida et al. 1993). Channels formed by leptomeningeal cells in the subarachnoid space join nasal lymphatics, and when tracers are injected into the cisterna magna in the rat, they drain to lymph nodes in the neck in less than 1 min (Kida et al. 1993). Although there are arachnoid villi in the rat, they are small and mostly associated with the dorsal aspect of the olfactory bulbs (Kida et al. 1993). It is estimated that at least 50 % of cranial CSF in the rat drains to cervical lymph nodes by the nasal route, and this has implications for immunological reactions in the central nervous system (Cserr and Knopf 1992; Weller et al. 2010).
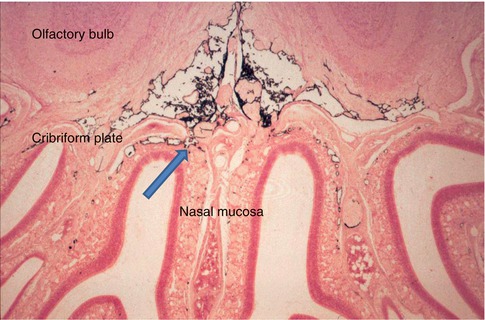

Fig. 3.5
Coronal section through the olfactory bulbs , cribriform plate and nasal mucosa of a rat that had received an injection of Indian ink into the CSF. Black Indian ink is seen in the subarachnoid space inferior to the olfactory bulbs. Channels cross the cribriform plate adjacent to branches of the olfactory nerve and allow Indian ink to drain into the lymphatics of the nasal mucosa (arrow) (Reproduced with permission from Kida et al. (1993)). Haematoxylin and eosin ×40
3.3.4 Drainage of Cerebrospinal Fluid via Arachnoid Villi and Granulations
In humans, the brain is much larger than in other mammals and the volume of CSF is much greater. Similarly, the sizes of arachnoid granulations and villi associated with the superior sagittal sinus and other cerebral and spinal sinuses are also much greater (Upton and Weller 1985). In humans, therefore, CSF drains directly back into the blood, via arachnoid granulations and villi, as well as through the cribriform plate and nasal lymphatics. However, the balance of amounts of CSF draining via these two routes is still unclear (Johnston et al. 2004).
Structurally, arachnoid granulations and villi are extensions of the leptomeninges, protruding through perforations in the dura mater, into venous sinuses (Fig. 3.6a) (Kida and Weller 1993; Kida et al. 1988). CSF appears to percolate through a mesh of collagenous trabeculae, which is coated by arachnoid cells and located in the centre of the villus or granulation. The CSF finally reaches the venous endothelium via channels in a compacted layer of leptomeningeal cells that caps each granulation or villus (Upton and Weller 1985) (Fig. 3.6b, c). Tracer studies in monkeys suggest that CSF then drains through the venous endothelial cells by a bulk flow mechanism that entails the transport of macrovacuoles across the endothelial cells (Tripathi and Tripathi 1974). Despite the large size of some arachnoid granulations, drainage of CSF appears to be restricted to an apical area some 300 μm in diameter (Upton and Weller 1985).
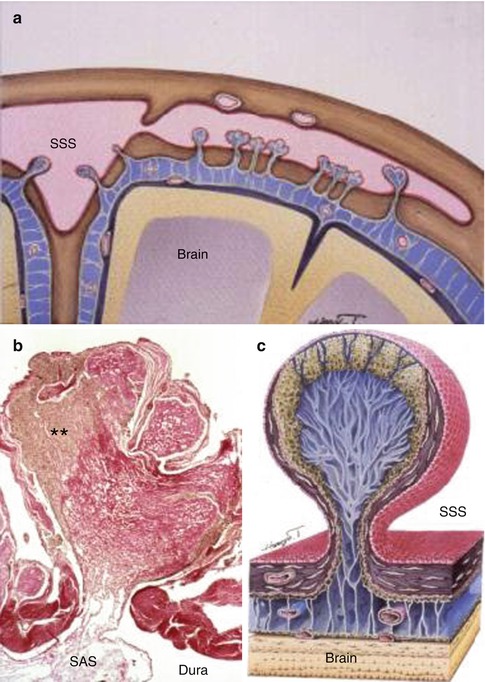

Fig. 3.6
Arachnoid granulations in the human brain. (a) Diagram showing the relationship between the brain, subarachnoid space (blue) and the superior sagittal sinus (SSS). Arachnoid granulations extend through the dura from the subarachnoid space into the superior sagittal sinus and its lateral extension. (b) Histological section through the length of an arachnoid granulation. CSF from the subarachnoid space (SAS) passes into channels in the collagenous core (red) of the granulation and then through channels in the arachnoid cap of the granulation (**) to reach the endothelium lining the venous sinus. Haematoxylin van Gieson ×40. (c) Diagram showing the main features of an arachnoid granulation with channels leading from the subarachnoid space to the endothelium (red) of the superior sagittal sinus (SSS) (Reproduced with permission (a, c) from Dr. Shinya Kida and (b) from Upton and Weller (1985))
3.3.5 Other Routes of Drainage of the Cerebrospinal Fluid
In addition to these major drainage pathways for CSF, through venous endothelium and via nasal and spinal lymphatics, some may also be absorbed directly into blood vessels in periventricular tissue (Johnston et al. 2004).
3.4 Interstitial Fluid (ISF)
In common with most other tissues of the body, the central nervous system has interstitial fluid within the extracellular spaces. In contrast with most other tissues, there are no conventional lymphatics in the central nervous system to drain interstitial fluid to regional lymph nodes. However, there are well-defined perivascular lymphatic pathways in the CNS by which interstitial fluid and solutes drain from the brain and spinal cord. These run along the basement membranes in the walls of capillaries and arteries, to reach regional lymph nodes (Weller et al. 2009b). Perivascular lymphatic drainage of interstitial fluid appears to be largely separate from the CSF drainage, with only 15 % of ISF leaking into the CSF (Szentistvanyi et al. 1984). This is contrary to some established concepts that the CSF acts as a sink for metabolites from the brain, a concept that needs to be re-examined in the light of more recent work on the drainage of interstitial fluid (Carare et al. 2008; Weller et al. 2009b).
3.4.1 The Blood–brain Barrier and Production of Interstitial Fluid
The blood–brain barrier (BBB) is one of the major systems that regulate homoeostasis and the constancy of the neuronal environment in the CNS. The BBB is located in the capillary endothelial cells of the brain and spinal cord and appears to be induced by the presence of perivascular astrocytic and neuronal processes; it is characterised by the tightness of intercellular junctions and the relative absence of trans-endothelial vesicular transport (Fig. 3.7a) (Abbott et al. 2006; Nag et al. 2011). Although water may pass freely across the blood–brain barrier, other molecules are actively transported from blood to brain; many substances are blocked from entering the CNS by the blood–brain barrier.
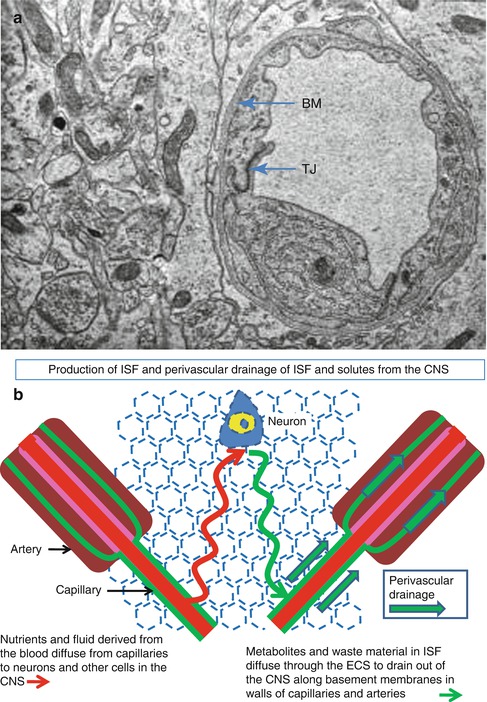

Fig. 3.7
Production and drainage of interstitial fluid . (a) Transmission electron micrograph of a capillary and surrounding brain tissue in the human cerebral cortex. Endothelial cells, joined by tight junctions (TJ), surround the lumen of the capillary and are coated on the abluminal surface by basement membrane (BM). Neuronal and glial processes are tightly packed together and separated by a very narrow extracellular space. ×14,000. (b) Diagram to show the passage of fluid and soluble nutrients from a capillary, through the narrow extracellular spaces, to neighbouring neurons (red line) and the drainage of fluid and soluble metabolites out of the brain along perivascular basement membranes in the walls of capillaries and arteries (green line) (Reproduced with permission (a) from Preston et al. (2003))
The volume of interstitial fluid in the human brain has been estimated at approximately 280 mL (Bergsneider 2001). It is produced partly from the blood and partly from the metabolites produced by the CNS tissue itself. The estimated range of drainage of ISF is 0.11–0.29 μL/min/g of tissue (Abbott 2004); this is comparable with the drainage of ISF from other organs (Szentistvanyi et al. 1984). Although the study of ISF has been largely overshadowed by concentration of research on CSF, the production and drainage of ISF has implications particularly for neurodegenerative disorders, neuroimmunological diseases (Weller et al. 2010), hydrocephalus and syringomyelia.
Fluid in the central nervous system is increased in three main types of oedema. Cytotoxic oedema occurs in the very early stages of damage to the CNS, particularly in grey matter, when cells are deprived of oxygen or glucose and die (Marmarou 2007). As ATP production ceases, ion pumps at the cell membrane no longer function and allow the influx of sodium and other electrolytes into the cell, followed by water, with the result that cells swell and burst. Vasogenic oedema results from the breakdown of the blood–brain barrier following tissue damage in the CNS and the outpouring of fluid, proteins and other solutes into the brain tissue (Marmarou 2007; Nag et al. 2011). The third type is interstitial oedema (Weller 1998) due to the infusion of CSF into the white matter in hydrocephalus and syringomyelia (see Sects. 3.4.2 and 3.5.2). Accumulation of interstitial oedema fluid reflects the failure of interstitial fluid drainage pathways to accommodate increased ISF in the extracellular spaces of the CNS.
3.4.2 Circulation and Drainage of Interstitial Fluid
Fluid and nutrients cross the blood–brain barrier at the capillary endothelial cells and diffuse through the narrow extracellular spaces of the brain to supply neurons and glial cells (Abbott 2004; Abbott et al. 2006; Marmarou 2007) (Fig. 3.7a, b). Interstitial fluid and soluble metabolites then diffuse through the extracellular spaces (Syková and Nicholson 2008) to drain by bulk flow along the basement membranes in the walls of capillaries and arteries (Fig. 3.7b) within CNS tissue and leptomeninges (Carare et al. 2008; Weller et al. 2009b).
Evidence for perivascular lymphatic drainage pathways is derived from a series of experimental studies, initially using radioactive tracers that showed rapid elimination of interstitial fluid and solutes from the brain to cervical lymph nodes (Szentistvanyi et al. 1984). The detailed anatomy of the drainage pathway was later elucidated using fluorescent tracers and confocal microscopy (Carare et al. 2008). When fluorescent dextran is injected into grey matter of the mouse brain, it initially spreads diffusely through the extracellular spaces and then, within 5 min, is present in the basement membranes in the walls of capillaries and arteries in the brain and leptomeninges (Carare et al. 2008). It appears that interstitial fluid and solutes drain to the lymph nodes at the base of the skull from the walls of the carotid artery (Weller et al. 2009b). The motive force that drives perivascular drainage is thought to be the contrary, or reflection, wave that follows the pulse wave passing along cerebral artery walls; in this model, the contrary wave drives interstitial fluid out of the brain in the reverse direction to the flow of blood (Schley et al. 2006).
Although it is not possible to perform tracer studies in humans, there is one natural tracer that strongly indicates the presence of a perivascular lymphatic drainage pathway in the human brain, similar to that in the mouse. Amyloid -β (Aβ) is derived from amyloid precursor protein and is one of the peptides that accumulate within the brain in Alzheimer’s disease (Duyckaerts and Dickson 2011). Aβ also accumulates in the walls of capillaries and arteries in the brain and leptomeninges as cerebral amyloid angiopathy (Biffi and Greenberg 2011; Weller et al. 2009c, 2011) (Fig. 3.8a, b). The pattern of perivascular accumulation of Aβ is exactly the same as the distribution of fluorescent tracers defining interstitial fluid drainage pathways (Carare et al. 2008; Weller et al. 2009b). This strongly suggests that soluble Aβ is draining out of the brain along perivascular interstitial fluid drainage pathways (Biffi and Greenberg 2011; Weller et al. 2011). Furthermore, biochemical studies have shown that the accumulation of Aβ in the walls of the carotid arteries ceases at the base of the skull (Shinkai et al. 1995), suggesting that interstitial fluid with soluble Aβ drains from the artery wall to adjacent cervical lymph nodes in a similar way to that observed in experimental animals.
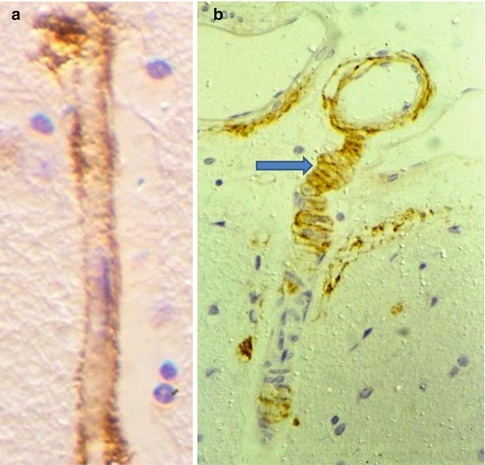

Fig. 3.8
Amyloid -β is deposited in the perivascular interstitial fluid drainage pathways in human brain as cerebral amyloid angiopathy. (a) Deposition of amyloid-β (brown) in the basement membrane surrounding a cortical capillary. Immunocytochemistry for amyloid-β ×750. (b) Leptomeningeal artery (top) extends a branch into the cerebral cortex. Amyloid-β (brown) is deposited in the basement membranes between the smooth muscle cells of the tunica media (arrow) suggesting that soluble amyloid-β drains out of the brain along perivascular pathways. Immunocytochemistry for amyloid-β ×200 (Reproduced with permission (a) from Preston et al. (2003) and (b) from Weller et al. (1998))
Although it is mainly the brain that is affected by amyloid angiopathy in Alzheimer’s disease, amyloid also accumulates in artery walls in the spinal cord in the familial British dementia (Revesz et al. 2009). This suggests that interstitial fluid and solutes drain from the spinal cord along perivascular pathways by a system similar to that observed in the brain.
3.5 Pathology of the Cerebrospinal Fluid
Pathology of the CSF falls into two main categories (Weller 1998):
(a)
Meningitis and haemorrhage in which inflammatory cells, erythrocytes or tumour cells are released into the CSF following infection, subarachnoid haemorrhage and invasion of the subarachnoid space by primary or metastatic tumours.
(b)
Obstruction to the flow of CSF that results in hydrocephalus in the brain and syringomyelia in the spinal cord.
3.5.1 Meningitis and Subarachnoid Haemorrhage
Bacterial and fungal infections of the subarachnoid space result in an outpouring of polymorphonuclear leucocytes and protein from the blood into the CSF (Brown and Gray 2008; Weller 1998). Inflammatory cells remain mostly confined to the subarachnoid space and do not, in general, invade the underlying brain or spinal cord tissue. The pia mater, subpial collagen and the tightly packed astrocyte processes, which form the glia limitans on the surface of the brain and spinal cord, appear to act as a barrier to the entry of infection and inflammatory cells into the CNS. Often the only reaction at the surface of the brain is proliferation of microglia. In tuberculous meningitis , however, caseating granulomata not only involve the leptomeninges but also extend into the surface of the brain or involve cranial nerve roots; there is invasion of the CSF by lymphocytes and high protein levels may be attained in the CSF (Brown and Gray 2008). One of the major complications of both pyogenic and tuberculous meningitis is inflammation in the walls of leptomeningeal arteries, thrombosis of their lumina and infarction of the underlying CNS tissue (Brown and Gray 2008). In the long term, bacterial meningitis may result in fibrosis of the leptomeninges, interfering with drainage of cerebrospinal fluid and resulting in hydrocephalus and syringomyelia.
In viral infections and in autoimmune disease, such as Guillain-Barré syndrome, there is a rise in the level of protein and the presence of lymphocytes in the CSF, indicating a breakdown in the blood-CSF barrier . The major complications of these conditions are not so much in the CSF but result from the involvement of brain tissue (encephalitis) or involvement of cranial and spinal nerve roots (autoimmune neuritis) (Weller 1998).
Subarachnoid haemorrhage results from rupture of a saccular aneurysm or an arteriovenous malformation or may follow an episode of trauma (Ferrer et al. 2008). Fresh arterial blood floods into the subarachnoid space and may spread widely over the surface of the brain and spinal cord and fill the cisterns at the base of the brain. Frequently there is extension of the haemorrhage into the brain itself resulting in a fatal intracerebral haemorrhage. If the patient survives the initial episode, the arteries that are surrounded by blood in the subarachnoid space may go into spasm resulting in cerebral infarction. The long-term effects of the subarachnoid haemorrhage may be fibrosis of the leptomeninges, disturbance of CSF drainage and hydrocephalus (Ferrer et al. 2008).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







