CHAPTER 154 Anatomy and Physiology of Pain
Primary Afferent Nociceptors
Nociceptive information is conveyed from the periphery to the spinal cord by small myelinated and unmyelinated primary afferents that travel through the dorsal root to synapse in the dorsal horn. As described in detail in Chapter 158, recent years have seen an explosion of information about the mechanisms of nociceptive signal transduction and the properties of primary afferents. Individual afferents manifest a high degree of specificity, with nociceptive neurons differentiated from low-threshold afferents in terms of physiology, morphology, and neurochemistry. The primary afferent fibers also exhibit significant plasticity in response to tissue conditions, with alterations in neuronal phenotype and enhanced responsiveness during inflammation or in response to damage to the nerve itself. This “primary afferent sensitization” is now recognized to be an important contributor to hyperalgesia and abnormal pain states.
Dorsal Horn and Ascending Pathways
Nociceptive afferents enter the dorsal horn and terminate both superficially (in laminae I and II) and more deeply (in laminae V, VI, and VII), as well as around the central canal. By contrast, low-threshold tactile afferents spare the superficial laminae and send their terminals primarily to laminae III and IV (sometimes called the nucleus proprius).1 The different terminations of nociceptive and low-threshold input to the dorsal horn highlight the link between anatomic and functional organization of nociceptive and non-nociceptive somatosensory pathways and extend the specificity seen at the level of primary afferents to the central nervous system. Second-order neurons in the nociceptive pathways are found primarily in the superficial layers and more deeply in laminae V and VI of the dorsal horn and are usually divided into two classes: wide dynamic range (WDR) and nociceptive specific (NS).2,3 WDR neurons receive convergent input from both nociceptive and non-nociceptive primary afferents. They consequently exhibit low thresholds within the innocuous range but, unlike low-threshold tactile dorsal horn neurons, code stimulus intensity through the noxious range. WDR neurons are distributed somatotopically within the dorsal horn, and although the receptive fields are relatively large, they are not so large that a contribution to stimulus localization is precluded. WDR neurons are more common in the deeper dorsal horn but are also found more superficially in laminae I and II. NS neurons do not receive non-nociceptive input and respond exclusively to noxious stimuli carried either by Aδ mechanoreceptors or by both Aδ and C nociceptors. Their receptive fields are small, which points to an important role in stimulus localization. They are concentrated in the more superficial layers.
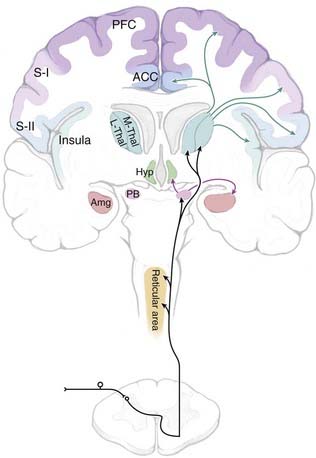
Nociceptive information is conveyed to the brain primarily via the spinoreticular, spinomesencephalic, spinoparabrachial, and spinothalamic tracts, all of which ascend through the anterolateral quadrant (Fig. 154-1). The importance of the anterolateral systems in pain (and temperature) sensibility is confirmed by the ability of anterolateral chordotomy to relieve pain, at least in the short term, in both patients and experimental studies.1 The spinothalamic tract also carries non-nociceptive information (innocuous warming and cooling, innocuous tactile information). In addition, tactile information is conveyed by the spinocervical tract and through the dorsal columns (which include ascending branches of large-diameter low-threshold primary afferents, as well as the postsynaptic dorsal column system, which consists of second-order projections of low-threshold dorsal horn neurons).
Direct spinothalamic projections terminate in both the medial and lateral thalamus, and these two targets can be considered more important in the affective-motivational and sensory-discriminative aspects of pain, respectively.4,5 Parallel spinoreticular and spinomesencephalic pathways may contribute to conscious sensation, but these pathways may be more important for arousal, autonomic and motor responses to noxious input, and recruitment of descending control systems (see the later section “Descending Modulatory Systems”). The spinoparabrachial pathway consists of projections of neurons primarily in lamina I and relays information to the amygdala and hypothalamus, as well as the midbrain periaqueductal gray (PAG) matter and caudal ventrolateral medulla. The spinoparabrachial pathway is considered particularly important in the emotional and autonomic aspects of pain.6
Role of the Dorsal Column Pathway in Visceral Pain
The classic view that ascending pathways through the dorsal columns are involved exclusively in the transmission of non-noxious information has recently been called into question by evidence that a postsynaptic dorsal column component contributes to visceral pain sensibility.7 These studies were motivated by the clinical observation that midline myelotomy relieves pain in patients with cancer involving pelvic visceral structures. Experimental studies subsequently documented a significant projection ascending ipsilaterally through the dorsal columns and transmitting information to the ventroposterolateral nucleus of the thalamus. Importantly, this pathway may not contribute to pain sensation under normal conditions but could become sensitized by visceral inflammation.8,9
Supraspinal Nociceptive Targets
Thalamus
The thalamus has been proposed to be involved in pain since as far back as 1911. Indeed, at that time pain was considered uniquely primitive among sensory systems and as being perceived at the level of the thalamus, without important cortical involvement.10 It is now recognized that reentrant interconnections between different thalamic nuclei and cortical areas set the stage for conscious perception of pain.11–14
When considering nociception, the thalamus is generally separated into medial and lateral aspects. Although with recent discoveries this division is no longer as clear-cut as was once thought, the lateral system is most strongly linked to the processing of sensory-discriminative information related to pain, whereas the medial system is more closely associated with emotional aspects of pain. The lateral system encompasses the ventral and posterior nuclei of the thalamus and their linkages with the lateral somatosensory cortices. The lateral thalamic nuclei receive direct spinothalamic input from both superficial and deep layers of the dorsal horn.15,16 Thalamic nuclei assigned to the lateral system include the ventral posterior medial (VPM) and ventral posterior lateral (VPL) nuclei, as well as the ventral posterior inferior (VPI) nucleus. Together, the VPM and VPL nuclei in humans are referred to as the ventrocaudal (Vc) nucleus. Medial and intralaminar nuclei along with their targets in the anterior cingulate cortex (ACC) and the medial prefrontal cortex (PFC) have been thought to constitute the medial pathway. In a recent and still controversial remapping of these systems, Dostrovsky and Craig have argued that a novel, primate-specific nucleus, the posterior portion of the ventromedial (VMpo) nucleus, represents the main thalamic relay for sensory-discriminative information, with the insula as the principal cortical target.13 The validity of this framework remains a topic of debate,15–19 and the controversy probably reflects the somewhat artificial nature of the medial/lateral pain system concept. Certainly, it should be recognized that these divisions are not as well demarcated as was once thought, and overlap and interconnections must exist in function and structure.
Lateral Thalamic Nuclei
Ventral Caudal Nucleus
The structures of the lateral thalamus primarily associated with nociception are the three nuclei that make up the Vc nucleus, also known as the principal sensory nucleus of the thalamus in humans or the ventral posterior (VP) complex in primates. In both humans and primates, the constituent nuclei are the VPM and VPL, which are functionally matched. Spinothalamic projections to the VPL nucleus arise from the spinal dorsal horn, whereas input to the VPM nucleus comes from the spinal trigeminal nucleus (i.e., the “medullary dorsal horn”).20–22 Spinothalamic/trigeminothalamic terminations are interdigitated with terminals of the medial lemniscal pathway, which carries somatotopically organized tactile information through the dorsal columns and to the dorsal column nuclei.23,24 The spinothalamic terminations in the VPL nucleus arrive in clusters and are roughly somatotopically organized in concordance with the same medial-lateral somatotopic organization as the lemniscal pathway.25–27
The output of the Vc nucleus is primarily to sensory cortical areas, most notably to the primary somatosensory (SI) cortex. Via retrograde tracing in primates, it was found that the Vc nucleus provides the majority of thalamic input to the SI cortex.28 Importantly, as many as 90% of identified nociceptive neurons of the VPL nucleus could be activated antidromically from the SI cortex.29 Other identified targets of Vc projections include the secondary somatosensory (SII) cortex, insula, and posterior parietal cortex.30–33
In addition to anatomic approaches, recording and stimulation of Vc neurons provides evidence for involvement of the region in sensory and discriminative aspects of pain. The majority of neurons in this region respond to innocuous or low-threshold mechanical stimuli, but as many as 10% are activated by noxious stimuli or changes in temperature.34–37 Clusters of these nociceptive-responding neurons can be identified most consistently near the posterior and inferior aspect.23,26,29,38,39 Neurons analogous to the NS and WDR classes first described in the dorsal horn can be identified.40 The Vc nucleus receives visceral as well as cutaneous input, and neurons in this nucleus have been shown to respond to noxious visceral stimuli, although the organization of responsive neurons is not apparently viscerotopic.41,42
Stimulation of the Vc nucleus in humans most often produces contralateral, nonpainful paresthesias even at higher intensities, at least in patients without a chronic pain complaint, although temperature and pain sensations can be evoked from some sites at the lowest stimulus intensity.43,44 Stimulation sites near the posterior and inferior border were significantly more likely to evoke painful or thermal sensations than were those in the core region.43 Lenz and colleagues45,46 have been able to distinguish stimulation sites at which a binary (pain versus no pain) sensation can be elicited and others at which an analog sensation, in which the sensory magnitude is related to stimulus intensity, is produced. They suggest that the information conveyed at sites associated with binary signaling are related to an “alarm” aspect of pain processing whereas processing at analog signaling sites is more important for coding stimulus intensity.
The effects of lesions and inactivation of the Vc nucleus further support the idea that this nucleus is a functional relay for nociceptive information. Focal application of lidocaine in this region in nonhuman primate results in reduced detection of small changes in skin temperature in the noxious range.47 In humans, lesioning of the Vc nucleus and the lateral thalamus more broadly has been attempted in the treatment of neuropathic pain, with resulting decreases in contralateral detection of thermal and mechanical pain, as well as touch and proprioception.48–50 However, such surgeries are necessarily approached with caution because of the risk of triggering iatrogenic central pain.51,52
Ventralis Caudalis Parvocellularis Nucleus
The human ventralis caudalis parvocellularis (Vcpc) nucleus is thought to be analogous to the region referred to as the VPI nucleus in primates.53 Although not as well studied as the Vc nucleus, this region is strongly associated with nociception, and one of its functions is as a relay nucleus for nociceptive signals. Primate studies show that input to the VPI nucleus travels via the spinothalamic tract and arises from neurons in laminae I, IV, and V.25,54,55 The VPI nucleus importantly differs from the neighboring VP nucleus in that the former projects primarily to the SII and insular cortices, both of which are involved in the perception of pain.56 Although the VPI nucleus is anatomically contiguous with the VPL/VPM regions, it is functionally distinct and has been tied to both the sensory and affective components of pain.57 Recording studies in both humans and primates have found numerous neurons in this region that respond specifically to the application of noxious stimuli.36,58,59 In humans, stimulation of the Vcpc nucleus apparently elicits painful sensations more reliably than does stimulation of the Vc nucleus itself.43,60
Posterior Part of the Ventral Medial Nucleus
The VMpo nucleus is the most recently identified of the pain-related nuclei of the lateral thalamus and remains a topic of significant debate.16–19 First identified by Craig and colleagues in primates on the basis of direct spinothalamic input from lamina I,55,61,62 the VMpo nucleus is found in the caudal aspect of the thalamus and is in a location that was formerly included within the posterior complex. A similar region posteromedial to the Vc nucleus has since been identified in humans.63 No analogue has been identified in nonprimate species. The VMpo nucleus projects primarily to the insula, with additional projections to the SI cortex.55
This area is of particular interest because from current evidence it appears to be a specifically nociceptive region of the thalamus. In initial recordings of neurons from primate VMpo nuclei, 97% of the neurons characterized were found to respond to either thermal or noxious stimuli. Further studies have confirmed the presence of WDR, NS, and thermoreceptive-specific neurons and the lack of low-threshold neurons. The recorded neurons have an anterior-posterior topographic organization and small receptive fields.55 Subsequent electrophysiologic recordings in awake human patients found that neurons in a region tentatively identified as the VMpo nucleus respond to innocuous or noxious cooling of the skin and that stimulation of the region elicits perceptions of cold or cooling.64 These authors suggest that the VMpo nucleus could be important not specifically as a nociceptive relay but rather for cooling/cold information.
Notably, however, debate has arisen regarding the location, identification, and characterization of the primate and human VMpo nucleus. Arguments regarding the projection of lamina I neurons in the VMpo nucleus are based on questions of identifying terminals versus fibers of passage. Using a different antibody to the same antigen, another group failed to replicate the staining results that first identified the VMpo nucleus in primates, and the human VMpo nucleus was identified primarily on the basis of antibody staining against the same antigen.16,17,19,53 In a study of poststroke lesions in humans, the authors found that poststroke pain and changes in temperature perception still occurred in the absence of VMpo damage, although lesions not including a significant part of the Vc nucleus did not cause these symptoms.52 Further research is needed to confirm previous studies on the boundaries and connectivity of the VMpo nucleus in primates and to characterize the physiology and responsiveness of VMpo cells in humans and primates.
Medial Thalamic Nuclei
Intralaminar Nuclei
The intralaminar nuclei have long been considered part of a “nonspecific” medial complex that sends diffuse projections to the entirety of the cerebral cortex.65 Intralaminar nuclei postulated to be involved in nociception include the central lateral (CL), center median (CM), and parafascicular (Pf) nuclei, with the Pf and CM nuclei sometimes considered together as the CM-Pf complex. Nuclear boundaries appear to be well matched among species, with only few significant differences. One of the primary interspecies differences in intralaminar nuclei is in their input. In humans, the CL is the only one of the three nuclei that receives spinothalamic projections, whereas in primates there are additional spinothalamic connections to the CM and Pf nuclei.21,22,66–68 Input to the CL nucleus arises from deeper aspects of the spinal gray matter, laminae V and VII.20,69,70 Output from the intralaminar nuclei goes diffusely to the cortex, including the posterior parietal and motor cortex. However, organized connections with striatal structures suggest that these nuclei convey important motor responses to noxious input.71–75
Recordings made in intralaminar nuclei support the idea that they are involved in processing nociceptive information, although their specific role still remains unclear. Neurons responding only to stimuli of noxious intensity have been identified in humans and primates in all three nuclei and in general have large, bilateral receptive fields.76,77 Recordings from the CM-Pf complex have shown that a large proportion of neurons respond to pinprick or noxious heat but none to non-noxious stimuli.78,79
Lesioning of the medial thalamus has been performed for intractable or neuropathic pain and has generally had positive results. In one study of 69 patients with neurogenic pain, medial thalamotomy was found to relieve the pain in 67%.79,80 Stimulation of the intralaminar nuclei includes pain and thermal sensation, along with unpleasant sensations such as dyspnea and dizziness.50,81,82
Ventral Caudal Part of the Medial Dorsal Nucleus
This area of the medial thalamus is often grouped with the intralaminar nuclei because of similarities in afferents and physiology, although the ventral caudal portion of the medial dorsal (MDvc) nucleus has received additional attention as a nociceptive relay after it was shown to receive a spinothalamic projection from lamina I of the spinal cord.16,55 Output from the MDvc nucleus is argued to be primarily to the ACC and frontal lobe. Based on these projections and on identification of NS neurons localized to the MDvc nucleus, this region is proposed to be important in the motivational aspects of pain.
Brainstem
The spinoreticular pathway arises in deeper laminae of the dorsal horn and sends projections to the medial and lateral brainstem core areas, including the lateral reticular nucleus, nucleus reticularis dorsalis, nucleus gigantocellularis, and “rostral ventromedial medulla” (RVM), and to the internal parabrachial nucleus (see Gauriau and Bernard6 for an up-to-date review). These connections are probably important in somatomotor integration of nociceptive responses, recruitment of descending modulatory systems, and engagement of arousal mechanisms.
The spinoparabrachial pathway, which consists of projections from lamina I to the lateral parabrachial region, is receiving increasing attention for its role in pain and especially chronic pain.6 The majority of neurons in the lateral/external parabrachial area are strongly activated by noxious stimulation over wide areas of the body. The nociceptive portion of the parabrachial complex projects heavily to the central nucleus of the amygdala and to the ventromedial hypothalamus. The connection through the amygdala to the extended amygdala has been implicated in emotional reactions to painful stimuli, and this input through the spinoparabrachial system is probably reinforced by direct projections to the amygdala from deeper spinal laminae, which have been demonstrated in both rodents and primates.83,84 The connection from the nociceptive parabrachial complex to the hypothalamus seems more likely to be related to motivated behavior triggered by pain, including defensive behavior, flight, and aggression.
Spinomesencephalic pathways terminate primarily in the lateral and ventrolateral PAG and are thought to be important in active versus passive coping strategies for dealing with escapable and inescapable pain, respectively.85,86 Such input presumably also triggers descending control mechanisms (see “Descending Modulatory Systems” later).
Cortical Processing
The inability to identify a specific cortical “center” that when lesioned eliminates pain gave rise to an idea that pain was uniquely primitive among sensory systems in being processed entirely at subcortical levels.10 However, the advent of functional imaging approaches reawakened interest in the cortical representation of pain, and the notion that the cerebral cortex is uninvolved in detection of noxious stimuli has since been discounted. It is now appreciated that there is not a single cortical “pain center” that mediates all aspects of the complex sensation that is pain but that a reasonably well-defined cortical network is recruited by acute noxious stimulation. Notably, although no lesion at a single site can eliminate the perception of pain, stimulation of any one of many sites can elicit painful perceptions, in conjunction with or independent of other somatosensory sensations. Such stimulation experiments along with recent advances in both noninvasive and invasive techniques has shed light on the contributions that the numerous cortical regions make to the “pain matrix.” Importantly, the cortical representation of long-standing chronic pain adds new layers of complexity to this picture.
Primary Somatosensory Cortex
The SI cortex is part of the anterior parietal lobe and forms the postcentral gyrus. The role of the SI cortex in somatosensory and mechanosensory function is well documented elsewhere.87,88 This region is thus well suited for the proposed role in nociception of discriminating location and intensity, although its specific role has been debated.31,89,90
The primary thalamic input to the SI cortex is via the VP nuclei of the thalamus, which as noted earlier receives both spinothalamic and lemniscal projections. In monkeys, nociceptive input to the SI cortex has been demonstrated,28 and studies in rats have also shown clusters of nociceptive neurons in this region of cortex.91 Nociceptive neurons in the SI cortex are much rarer in primates, however, and although some reports mention their existence, mechanosensory neurons are far more common.92 Nonetheless, subdural and other recording methods in humans have shown evidence of neurons specifically activated by noxious stimuli and specific activation of the SI cortex in pain-related tasks.93–95
A majority of recent imaging studies demonstrate activation of the SI cortex with experimental noxious stimuli, and SI activation has been linked specifically to pain intensity.96–98 Interestingly, a sustained noxious stimulation (immersion of the hand in hot water for 3 minutes under laboratory conditions) has been reported to give rise to decreased signal (blood flow) in this region.99
Despite the findings of nociceptive neurons and activation of the SI cortex in imaging studies, SI stimulation predominantly produces contralateral sensations of temperature, paresthesias, and other innocuous sensations; few, if any studies have reported sensations of pain evoked by stimulation of the human SI cortex.100–102 Studies of surgical and ischemic lesions in the SI cortex and surrounding areas have also shed light on its role in pain processing. Early reports of SI damage from head trauma pointed to hypoalgesia and deficits in spatial and temporal discrimination of noxious stimuli.103–105 A more recent case report of stroke-related damage to the right SI cortex described a patient’s difficulty in localizing laser-evoked noxious stimuli on the left side of the body; this patient was unable to discriminate the location of the stimuli further than to a single limb.106 However, surgical interventions in the SI cortex for pain control have been reported to generally be ineffective.107
Secondary Somatosensory Cortex
The SII cortex is located on the parietal operculum at the superior bank of the sylvian fissure, and although the functional specifics of pain processing are still being described, the SII cortex holds an unambiguous role in cortically mediated nociception. The SII cortex is proposed to be involved in recognition of painful and thermal stimuli, pain-related learning, and integration of tactile and nociceptive information. Thalamic input to the SII cortex comes largely from the VPI nucleus.108 Individual neurons in the SII cortex may be NS and tend to have large receptive fields with contralateral or bilateral activation.109
The SII cortex is consistently activated in imaging studies involving nociception. Its proximity to the temporal lobe has made it amenable to clinical research involving implantable, intracortical electrodes in patients with temporal lobe epilepsy, and a wealth of knowledge about function has been gained in recent years from such studies. With laser stimuli and implantable electrodes, SII responses were shown to be graded according to stimulation intensity, from the lowest sensory threshold to detection of stimuli as painful. Interestingly, however, the level of SII activation did not increase with increasing stimulation intensity above the threshold for detection of pain.110 In another study using depth electrodes, Frot and coworkers showed increased latency in SII activation from tactile input as compared with noxious stimuli, again supporting the idea of SII involvement in integration and learning.111 In imaging studies, the SII cortex is activated concurrently with or even before the SI cortex, thus indicating a parallel rather than a serial relationship between these two regions.112
Stimulation and lesion studies are few as a result of interpretative difficulties related to location and because ischemic lesions often extend into other areas. Only a few stimulation protocols have focused specifically on the SII cortex. One group reported that SII stimulation in humans did not invoke any painful sensations, although this work was focused primarily on the insula.113,114 In a systematic exploration of stimulation of the SII cortex, another group found the responses to be a mix of somatosensory, temperature, and painful sensations, with a similar percentage of evoked painful sensations as seen in the insula.102 Difficulties with location have also inhibited studies based on SII lesions, although in general, deficits in the SII cortex after lesions support the idea of its direct involvement in pain processing. In a case involving a pure sensory stroke involving only the SII cortex, the patient showed contralateral restricted deficits in light touch, pain, and temperature sensation.115 Other studies involving the SII cortex report central pain syndrome, hyperalgesia, and thermal and mechanical deficits but, notably, with parietal lesions that do not involve the SII cortex, no such changes are observed.116,117 Although some impediments to interpreting data related to the SII cortex exist, several lines of research unequivocally indicate the involvement of this region in pain processing and integration of tactile and nociceptive signals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree