19
Aneurysms of the Basilar Trunk: Far Lateral and Petrosal Approaches
Saccular aneurysms of the basilar artery have remained aloof, because of the presumed limitations of accessibility and fear of the consequences of changes in the basilar circulation. The surgical treatment of these aneurysms will undoubtedly develop in some degree and it would appear that in certain instances in which expectancy of life is poor, as with repeated hemorrhages, a direct attack is justifiable.
—Charles Drake, 19601
In the latter half of the 18th century, anatomist Cruveilhier described aneurysms of the vertebrobasilar system in his illustrations,2 and an angiographic demonstration of such aneurysms was first performed by Krayenbuhl. Though Dandy described his experiences with vertebrobasilar aneurysms, it was Schwartz who was credited with the first-ever preoperative diagnosis and direct surgical treatment of basilar artery aneurysm using a standard suboccipital craniectomy. Methods have included ligation of the neck of the aneurysm with or without excision of the aneurysm, trapping by ligation of arterial supply to the aneurysm, reinforcement of the aneurysmal wall, or ligation of the proximal vertebral artery in its intracranial or extracranial position. Standard subtemporal and suboccipital approaches have been used for many years. In 1959, Logue3 described the frontotemporal approach to basilar artery aneurysms. Drake1 compiled the experiences of neurosurgeons of the earlier years, and added his personal series of four cases in 1960. In this compiled series with 30 cases, there were only four deaths! Utilizing anatomic dissections, Drake exposed the upper third of the basilar artery and its bifurcation by an anterior subtemporal approach through the tentorial opening into the interpeduncular cistern. Brief periods of circulatory arrest helped to complete the dissection of the aneurysm neck. He also practiced division of the posterior communicating artery in some cases to improve access; however, not in all cases, as proposed by Gillingham.4 Kenneth Jamieson5 reported another series of 19 cases of vertebrobasilar aneurysms in 1964 with a mortality of 10 out of the 19. Taking their cue from Mullan et al6 and Stevenson et al,7 who approached anterior brainstem lesions via the transclival route, John Fox2 and Sano et al8 simultaneously described basilar craniectomy and transpharyngeal-transclival approaches for obliteration of a midline vertebral artery aneurysm. The technical difficulties of a narrow operating space combined with a high risk of infection made the approach less popular. Meanwhile, a large number of patients were operated upon by Drake9 utilizing the subtemporal route, and later Yasargil et al10 added the microsurgical pterional/transsylvian approach to reach the basilar tip.
Access to a midbasilar or low-lying basilar bifurcation, however, remained difficult, and Kasdon and Stein11 in 1979 proposed a combined subtemporal and suboccipital approach. Solomon and Stein12 reported their experience with this combined approach to reach low basilar and vertebral artery aneurysms with results superior to the natural history. The sigmoid sinus was divided inferior to the vein of Labbé, enabling retraction of the temporal lobe, whereas the cerebellum was lifted medially and superiorly to visualize the lateral side of brainstem below the petrous apex. This combined approach facilitated visualization of cranial nerves III through XI and the lateral side of the cerebral peduncle and pons. Kawase et al13 reduced retraction damage to the temporal lobe by advocating the extradural transpetrosal technique. However, this corridor remains constricted between the trigeminal nerve and the cochlea and has a potentially high risk of hearing loss and cerebrospinal fluid otorrhea. During the same period, Giannotta and Maceri14 reported performing a retrolabyrinthine transsigmoid craniectomy to reach aneurysms arising from the basilar artery trunk. The incision extended from the asterion to below the rim of the foramen magnum, thus reducing the more extensive incisions of the combined approach. The air cells are removed from an area bordered superiorly by the floor of the middle fossa and the superior petrosal sinus, inferiorly by the jugular bulb, and anteriorly by the posterior semicircular canal (SCC). Bone overlying the sigmoid sinus was removed, thus exposing the posterior fossa dura anterior and posterior to the sigmoid sinus. The sinus was later divided. The positive aspect of this approach is the familiarity of most surgeons with the suboccipital exposure of posterior fossa. For a shallower posterior approach to the clival region, Sekhar and Estonillo15 proposed a combined transcochlear and infratemporal approach to reach basilar trunk. Several modifications to the standard suboccipital route followed, and more so to the traditional approaches to cerebellopontine angle tumors in the form of transcochlear, translabyrinthine, transotic, and transmastoid extensions. Miller et al16 introduced a common nomenclature for all of these modifications: anterior and posterior transpetrosal approaches that would lead to lesions at the brainstem or clivus or the basilar trunk. The conventional suboccipital approach also received some extensions as Heros in 1986 described a lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. Further modifications by the extent of excision of the occipital condyle resulted in far-lateral and extreme lateral approaches.17,18
♦ Transpetrosal Approaches
An imaginary line drawn between the two internal auditory meatus would make a plane for two different topographic groups of lesions: anterior plus superior and posterior plus inferior. The anterosuperior would be lesions of the petrous apex and the superior half of the clivus that can be accessed by an anterior/temporal petrosal approach, and the posteroinferior would be lesions of the cerebellopontine angle and the petroclival and jugular fossa, which are approachable via a posterior transpetrosal route. The anterior approach could be combined with a temporal approach, whereas the posterior approach could be combined with a lateral suboccipital approach to improve visibility and access to the midline lesions.
♦ Anterior Petrosal Approach
This approach is performed with the patient in the supine position with shoulder elevation and the head kept in a horizontal plane. As a routine, a lumbar spinal puncture and cerebrospinal fluid (CSF) drainage facilitate brain relaxation and decrease the chances of a postoperative CSF fistula.
An anterior petrosectomy is performed through a subtemporal craniotomy, with a zygomatic osteotomy utilizing a Falconer’s flap preserving the superficial temporal artery. Wide exposure toward the midline is not necessary, whereas a wide basal tangent helps in manipulating instruments (Fig. 19.1). An extradural dissection (described by Kawase in detail) exposes the middle meningeal artery, lesser superficial petrosal nerve, and greater superficial petrosal nerve (GSPN) (Fig. 19.2). All these structures are divided by some surgeons, to further the dissection and avoid traction on the facial nerve. Drilling of the GSPN with diamond bur reveals the internal carotid artery in the Glasscock triangle. The geniculate ganglion can be identified by tracing the GSPN posteriorly or by opening the epitympanum, which lies posterolateral to the geniculate ganglion.
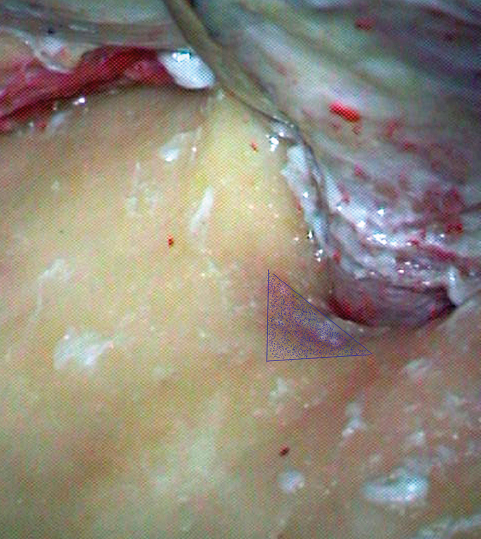
Fig. 19.1 The spine of Henle, serving as a landmark to the mastoid antrum (dotted blue triangle).
The arcuate eminence is a landmark for the superior SCC, which lies perpendicular to the petrosal ridge. The flat area of bone, between the geniculate ganglion and the arcuate eminence, forms the meatal plane, overlying the internal auditory canal (IAC). Initially, this soft bone is drilled to reach the hard conch of bone overlying the bony SCC inside the arcuate eminence. This drilling exposes the IAC anterior to the SCC and is continued anteriorly to open the internal auditory meatus to expose the posterior fossa dura (Fig. 19.3). At the opening of the IAC, a vertical bony spicule called Bill’s bar separates the superior vestibular and facial nerves. Bill’s bar is an important anatomic landmark to identify the cochlea, which is separated by the facial nerve from the ampulla of the SCC.
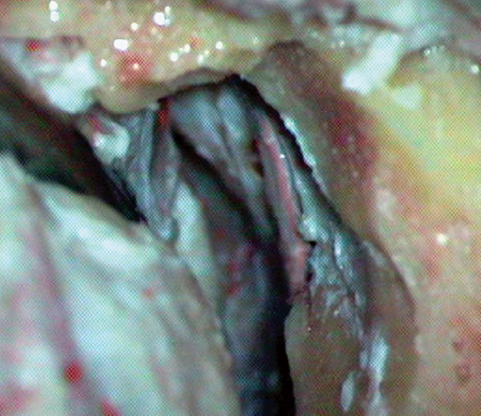
Fig. 19.2 The temporal fossa dissection exposed the arcuate eminence, which is the important landmark in the exposure.
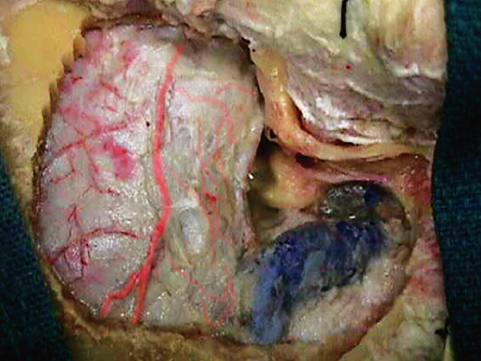
Fig. 19.3 The continuation of the middle fossa to the posterior fossa, through the drilled petrous bone.
The cochlea represents the posterolateral limit of the bony exposure through Kawase’s triangle and need not be exposed (Fig. 19.4). The hard otic capsule encasing the cochlea is a compact bone and looks distinctly lighter than the surrounding bone of the petrous apex. This remaining bone at the petrous apex bounded by the carotid artery, cochlea, and SCC/IAC constitutes the Kawase’s triangle and can be removed until the inferior petrosal sinus is exposed along the posterior fossa dura (Fig. 19.5).
The surgeon must keep in mind that extensive drilling along the petrous bone does not increase the visibility or exposure of the operative field and only increases the risk of injury to the cochlea, vestibule, internal carotid artery, and facial nerve, with subsequent neurologic deficits as well as CSF fistula and postoperative infection.
The middle fossa dura is usually opened along the temporal lobe. The tentorium is opened posterior to the trochlear nerve and lateral to the superior petrosal sinus. A perpendicular incision continues from here into the posterior fossa inferiorly.
♦ Posterior Petrosal Approach
The patient is placed in the lateral position with the head rotated horizontally to reduce venous obstruction at the neck. Placement of a lumbar drain precedes the positioning of the patient. The basic technique utilizes the mastoidectomy approach along the periauricular region with a sinuous curvilinear incision that can be extended superiorly, inferiorly, or in an inverted U-shaped incision (Fig. 19.6). A standard craniotomy with four bur holes located over the asterion, both sides of the superior nuchal line, and at the junction of the inferior temporal line and parietomastoid suture is performed followed by a mastoidectomy (Fig. 19.7). All the cortical bone overlying the mastoid is removed superiorly to the supramastoid crest, inferiorly to the tip of mastoid process, and anteriorly to the posterior wall of the external auditory canal, thus delineating the compact sigmoid plate overlying the sigmoid sinus and sinodural angle (Fig. 19.8). The mastoid antrum is identified following the middle fossa plate and external auditory canal posterior wall. Medially, the cortical floor of antrum is formed by the lateral semicircular canal (LSCC) and requires preservation. The inferior surface of the LSCC is close to the external genu of the facial nerve and does not need to be exposed. Removal of the mastoid air cells inferiorly would expose the digastric ridge, which would lead anteriorly to the facial nerve at the stylomastoid foramen and extracranially. Removal of air cells in the inferior part of the mastoid and retrofacial air cells between the antrum and digastric ridge exposes the fallopian canal (encasing the facial nerve). The posterior SCC runs parallel to the posterior fossa plate of bone and is bisected by the LSCC. It is located between the LSCC and the posterior fossa plate.
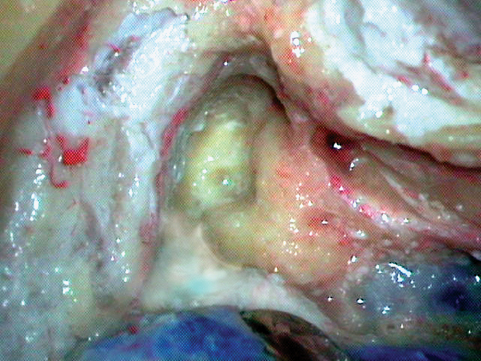
Fig. 19.4 Drilling between the geniculate ganglion and the arcuate eminence, over the meatal plate; the hard otic capsule can be seen with a different texture and color.
The sigmoid plate is followed inferiorly through the infralabyrinthine air cells to expose the jugular bulb. Only the beginning of the jugular bulb is exposed during the posterior petrosectomy, and exposure of the jugular bulb can be improved by skeletonization of the facial nerve in the fallopian canal. The superior SCC is perpendicular to the LSCC and can be exposed by following the sinodural angle through the supralabyrinthine air cells and through the dense cortical bone located along the petrous ridge at the juncture of the posterior and middle fossa bone plates (Fig. 19.9). In a combined approach, this step can be facilitated by removing the middle fossa bone flap and identifying the arcuate eminence from a subtemporal trajectory (vide supra).
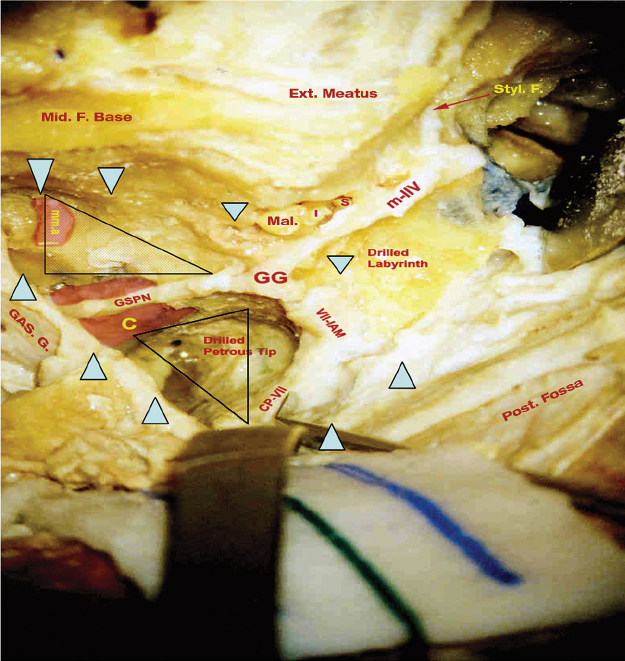
Fig. 19.5 Petrosal approaches. Intradural dissection in a cadaver, showing the drilled labyrinth and the petrous apex. The dotted area represents Glasscock’s triangle bounded by the greater superficial petrosal nerve (GSPN), the V3 nerve, and a line from the foramen spinosum to the facial hiatus. The thick blue arrowheads represent the extent of bone removed. The empty triangle represents Kawase’s triangle. C, internal carotid artery; CP, chorda (tympany) posterior; Ext., external; GG, geniculate ganglion; GAS. G., Gasserian ganglion; I, incus; IAM, internal acoustic meatus; mma, middle meningeal artery; Mal., malleus; Mid. F., middle foramen; Post. fossa, posterior fossa; S, stapes; Styl. F., stylomastoid foramen.
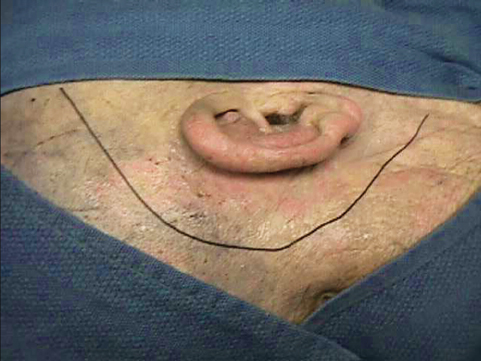
Fig. 19.6 A periauricular incision can be fashioned in a curvilinear or inverted-U shape, basically to meet the mastoidectomy requirement to reach the petrous apex along the internal auditory canal.
This area bounded by the posterior fossa dura, middle fossa dura, and posterior canal wall forms the Trautmann’s triangle, which actually represents the area of bone removed by the posterior petrosectomy approach (Fig. 19.10). The plates of bone overlying the middle fossa, posterior fossa, and sigmoid sinus are left behind until the end of the procedure to protect the dura and venous sinuses and guide the dissection. Elevating these bone plates completes petrosectomy. The medial portion of the petrous ridge remains deep to the superior SCC and posterior SCC, which can be drilled off as required, carrying the dissection across the IAC into the petrous apex.
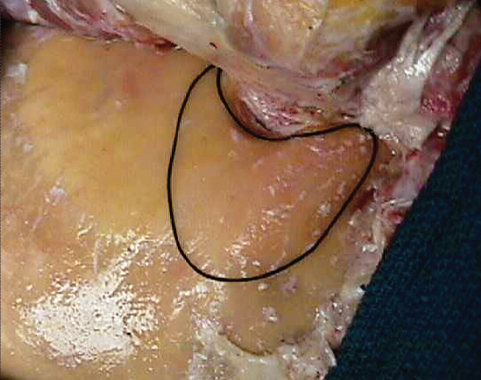
Fig. 19.7 The area of the mastoidectomy is marked on the surface of the bone.
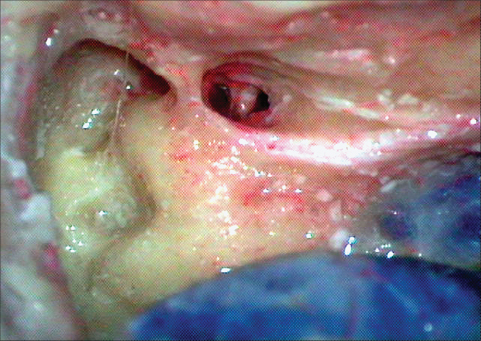
Fig. 19.8 As the cortical bone of the mastoid is removed to reach the suprameatal crest and along the posterior wall of the auditory canal, the compact bone overlying the semicircular canals and the sigmoid plate can be reached. The mastoid antrum can be seen close to the middle fossa dura, and the conchal bone posterior to it need not be disturbed. Over a period of time, we realized that excessive bone removal over the semicircular canals does not contribute to the exposure significantly.
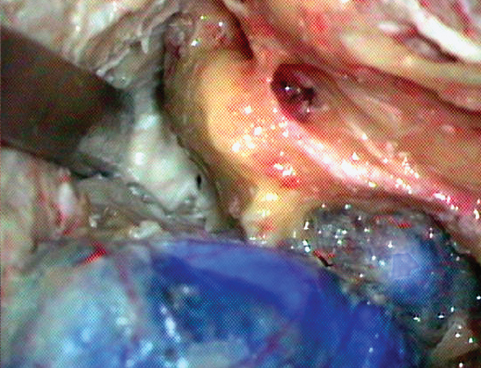
Fig. 19.9 As the bone anterior and medial to the semicircular canals is drilled away, the junction of the posterior and middle fossa bone plates can be reached, thus completing the petrous apicectomy.
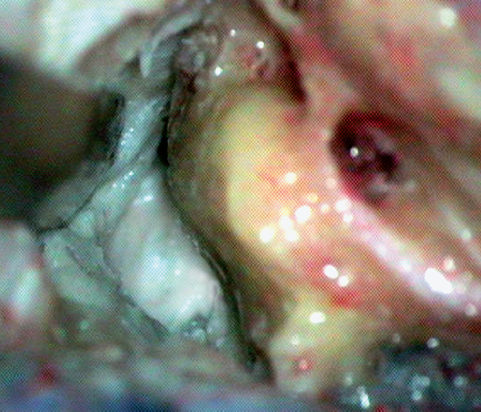
Fig. 19.10 The triangular area is the bone removed by the posterior petrosal approach, approximately representing Trautmann’s triangle. This exposes the posterior fossa dura medial to the posterior wall of the auditory canal.
The dural incision is made along the jugular bulb to the superior petrosal sinus, sparing the endolymphatic sac, and then along the posterior temporal lobe and anteriorly along the inferior temporal lobe. After dividing the superior petrosal sinus, the tentorium is cut posterior to the trochlear nerve (Fig. 19.11). Now the temporal lobe can be gently retracted along with the cut edge of the tentorium, avoiding injury to the cortex. The bulky nervous structure seen at this point is the trigeminal nerve, which is exposed while it enters Meckel’s cave (Fig. 19.12). Illustrative case angiograms are shown in Fig. 19.13.
An anterior petrosectomy provides access to the posterior fossa between the internal carotid artery, trigeminal root, and facial nerve. Lesions posterior to the IAC are better approached through a posterior petrosectomy or suboccipital craniotomy with preserved hearing. A posterior petrosectomy, in combination with as suboccipital-subtemporal craniotomy, opens up the entire posterior face of the petrous bone, the upper two thirds of the clivus, the anterior cerebellum, and the brainstem. Inferiorly, the anterior lip of the foramen magnum can be visualized. This lowermost exposure can be obtained by combining the posterior petrosectomy and the far lateral suboccipital approach.
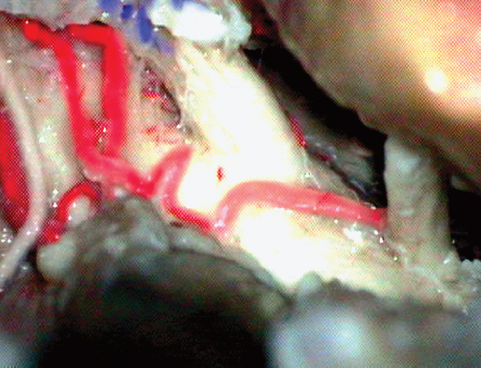
Fig. 19.11 As the tentorium is cut after dividing the superior petrosal sinus, the trochlear nerve is visualized and carefully dissected free away from the tentorial hiatus.
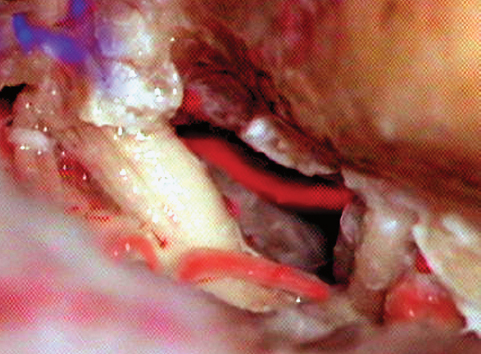
Fig. 19.12 Gentle retraction of the temporal lobe and the tacked-up tentorium now exposed the bulky fifth nerve entering Meckel’s cave. Dissecting the nerve along with the arachnoid and gentle retraction expose the basilar trunk, along with the anterior inferior cerebellar artery (AICA).
♦ Far Lateral Suboccipital Approach
Drake and Peerless19–21 used either the subtemporal transtentorial or the suboccipital approach for aneurysms of the vertebrobasilar (VB) system and enjoyed great success. The lateral suboccipital approach has been in vogue for vertebral artery and VB junction aneurysms. Conventional occipital bone removal supplemented by excision of the posterior arch of the atlas up to the sulcus arteriosus laterally provides adequate exposure for most lesions at the foramen magnum, whereas the vertebral artery, for the most part, is identified early in the operative procedure.
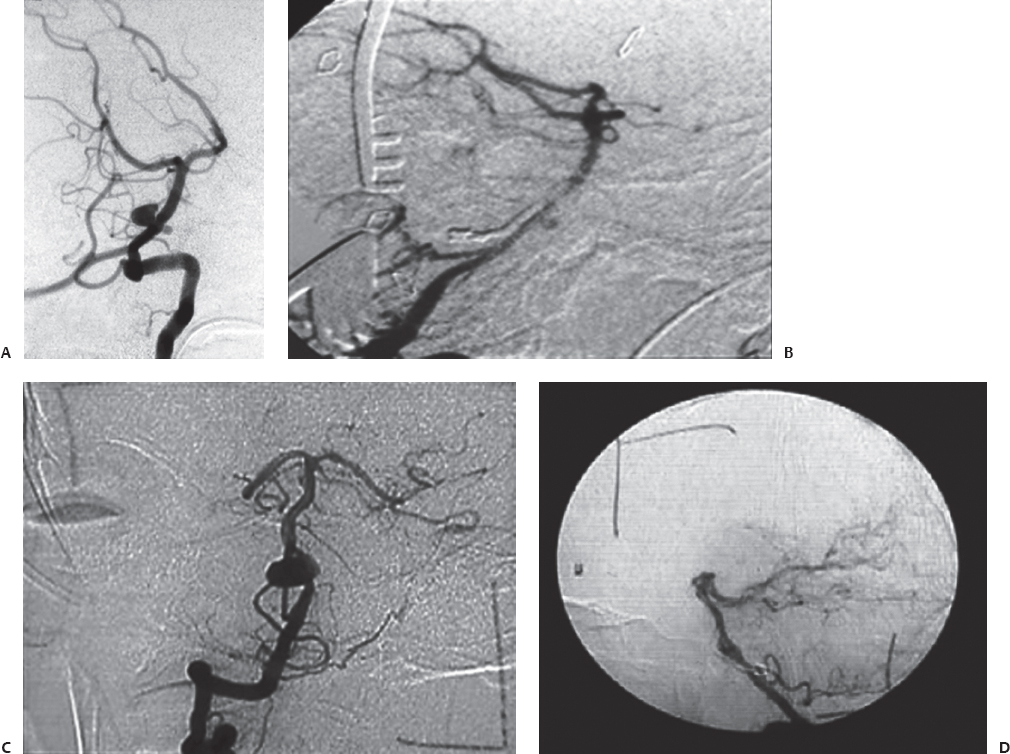
Fig. 19.13 (A–D) Preoperative and intraoperative angiograms showing successful obliteration of the basilar trunk aneurysms.
In his modest report, Heros22 described a gradual evolution of lateral suboccipital approach, later renamed and modified by many, as the far lateral, transcondylar suboccipital, and extreme lateral suboccipital approach with removal of the occipital condyle to a variable extent.17,18,22–25
The far lateral suboccipital craniotomy is performed in the lateral position. The patient is positioned under general anesthesia after placement of a lumbar drain. The incision is modified to a C-shaped retroauricular incision, one situated 2 cm medial to the mastoid, and reaching up to the C2 spinous process in the midline. This incision facilitates exposure of the mastoid and foramen magnum and the arch of C1 below. The muscles are divided with electrocautery along the line of the skin incision down to the suboccipital bone, where a subperiosteal dissection is performed. As the flap is reflected inferiorly, a sharp dissection is preferred to avoid injury to the vertebral artery at the foramen magnum (Fig. 19.14). The posterior arch of the atlas is exposed by continued subperiosteal dissection. A suboccipital craniotomy is performed after a single bur hole is placed over the occipital bone (to be replaced at the end of the procedure). Bone removal is now continued after excision of the posterior arch of the atlas toward the sulcus arteriosus. Bleeding from the robust venous plexus in this region can be avoided by placing cotton pledgets and bipolar dissection along the periosteum. Preferably now, the vertebral artery is identified with finger palpation along the lateral end of the foramen magnum. Thus, the most important aspect of this approach is adequate removal of bone in the area of the foramen magnum going laterally as far as the condylar fossa, posterior to the occipital condyle in close proximity to the entry of the vertebral artery into the dura (Fig. 19.15). At this lateralmost part of the foramen magnum, troublesome venous bleeding may be encountered, which can be controlled with bipolar coagulation and packing with oxidized cellulose. At the lateral extent of the exposure, the bone ridge becomes more vertical, and a high-speed drill is used to remove the last few millimeters of bone along the foramen magnum. This key point of the drilling enables exposure of anterior aspects of the medulla and upper cervical spinal cord from a lateral view without any retraction. As described by Heros, this lateral rim of the foramen magnum represents to the suboccipital exposure what the pterion does to the frontotemporal approach.
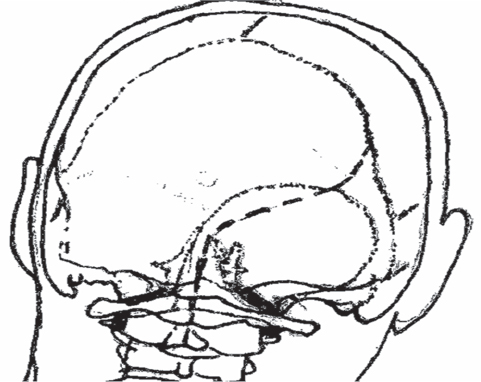
Fig. 19.14 An outline of the skin incision, the craniotomy, and the dural exposure, afforded by the far lateral approach. Note the exposure of the vertebral artery above the C1 arch; its point is an important surface landmark.
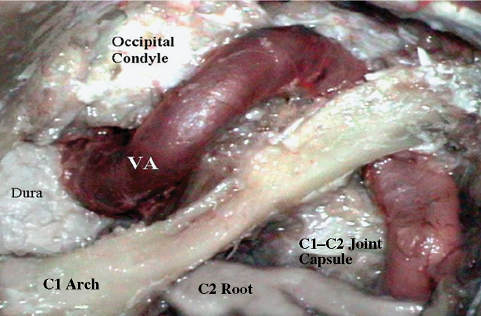
Fig. 19.15 The far lateral approach, landmarks, and arterial relationships. VA, vertebral artery.
The dura is opened with an oblique incision toward the midline at C1 and taking a cuff around the vertebral artery. The cuff of dura around the vertebral artery is essential for a watertight closure of the dura. The lateral flap of dura is tented up with sutures secured to the lateral muscle mass. Opening the cisterna magna and dividing the denticulate ligament assist in relaxation and gravity-aided retraction of the brainstem. Gentle retraction on the cerebellar tonsils, in the early phases, is required to visualize more medial structures. As the CSF drainage continues, the retractor may be removed soon, to reduce retraction injury of the cranial nerves and brainstem.
The vertebrobasilar junction can be approached inferolaterally, between the lower cranial nerves while tracing the vertebral artery superiorly. For a higher location, a working space can be safely created between the lower cranial nerves and the cranial nerve VII-VIII complex (Figs. 19.16 and 19.17). Case illustrations of the pre- and post-clipping angiograms can be seen in Fig. 19.18.
An extreme lateral transcondylar approach was described by Sen and Sekhar17 in 1990 for tumors in the region of the foramen magnum and later advocated for aneurysms of the vertebrobasilar junction by Sekhar et al26 in 1994. The authors recommended extensive excision of the occipital condyle and in some instances of the jugular tubercle to enhance visibility. In 2002, we found in our cadaver study, as applied to a clinical series, that more extensive bone removal of the occipital condyle did not significantly improve visibility.18 It has been the experience of many authors that the nature, extent, and location of the lesion determines the resectability rather than the extent of the condylar excision.27–30
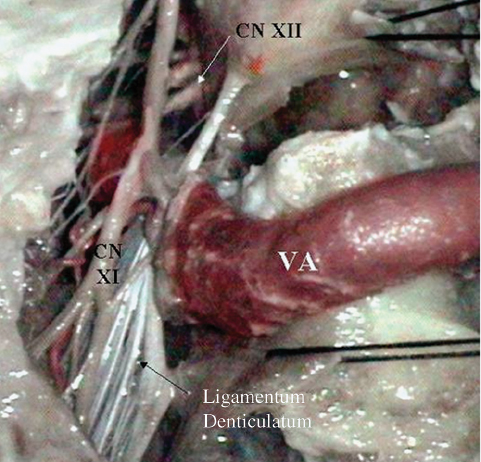
Fig. 19.16 The far lateral approach and intradural anatomy showing the lower cranial nerves (CN) and vertebral artery (VA).
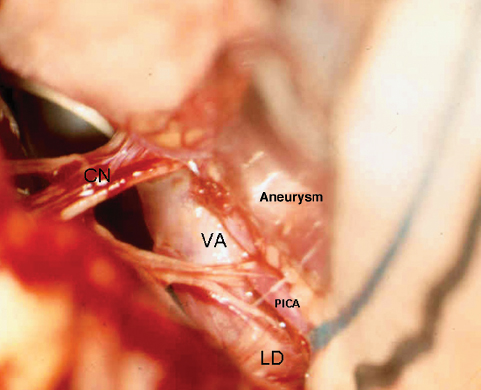
Fig. 19.17 The far lateral approach. An intraoperative photograph shows the vertebral artery (VA) and lower cranial nerves (CN) in a case of a posterior inferior cerebellar artery (PICA) aneurysm. LD, ligamentum denticulatum.
It is very appropriate to recall the philosophical statement by Robert Smith31 while performing cranial base surgery:
Only so much retraction of these structures (brainstem, cranial nerves and brain) is permissible. It is not the bony opening that limits an unobstructed view. Much of our current enthusiasm for cranial base approaches is based on what we can get away with, rather than what is needed, this too will pass.
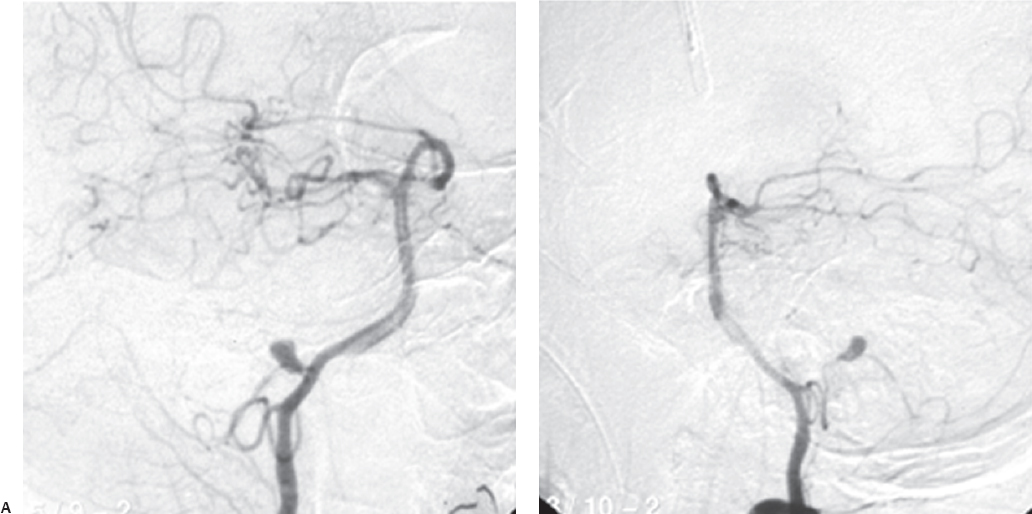
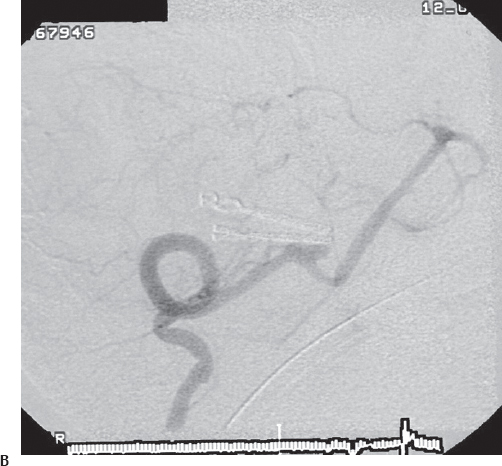
Fig. 19.18 Preoperative (A) and intraoperative (B) pictures of a successful obliteration of a PICA aneurysm.
References
1. Drake CG. Bleeding aneurysms of the basilar artery. Direct surgical management in four cases. J Neurosurg 1961;18:230–238 PubMed
2. Fox JL. Obliteration of midline vertebral artery aneurysm via basilar craniectomy. J Neurosurg 1967;26:406–412 PubMed
3. Logue V. Cerebral arterial disease. Trans Med Soc Lond 1959;75:161–173 PubMed
4. Gillingham FJ. The management of ruptured intracranial aneurysm. Ann R Coll Surg Engl 1958;23:89–117 PubMed
5. Jamieson KG. Aneurysms of the Vertebrobasilar System; Surgical Intervention in 19 Cases. J Neurosurg 1964;21:781–797 PubMed
6. Mullan S, Naunton R, Hekmat-Panah J, Vailati G. The use of an anterior approach to ventrally placed tumors in the foramen magnum and vertebral column. J Neurosurg 1966;24:536–543 PubMed
7. Stevenson GC, Stoney RJ, Perkins RK, Adams JE. A transcervical transclival approach to the ventral surface of the brain stem for removal of a clivus chordoma. J Neurosurg 1966;24:544–551 PubMed
8. Sano K, Jinbo M, Saito I. Vertebro-basilar aneurysms, with special reference to the transpharyngeal approach to basilar artery aneurysm. No To Shinkei 1966;18:1197–1203 PubMed
9. Drake CG. The treatment of aneurysms of the posterior circulation. Clin Neurosurg 1979;26:96–144 PubMed
10. Yasargil MG, Antic J, Laciga R, Jain KK, Hodosh RM, Smith RD. Microsurgical pterional approach to aneurysms of the basilar bifurcation. Surg Neurol 1976;6:83–91 PubMed
11. Kasdon DL, Stein BM. Combined supratentorial and infratentorial exposure for low-lying basilar aneurysms. Neurosurgery 1979;4:422–426 PubMed
12. Solomon RA, Stein BM. Surgical approaches to aneurysms of the vertebral and basilar arteries. Neurosurgery 1988;23:203–208 PubMed
13. Kawase T, Toya S, Shiobara R, Mine T. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg 1985;63:857–861 PubMed
14. Giannotta SL, Maceri DR. Retrolabyrinthine transsigmoid approach to basilar trunk and vertebrobasilar artery junction aneurysms. Technical note. J Neurosurg 1988;69:461–466 PubMed
15. Sekhar LN, Estonillo R. Transtemporal approach to the skull base: an anatomical study. Neurosurgery 1986;19:799–808 PubMed
16. Miller CG, van Loveren HR, Keller JT, Pensak M, el-Kalliny M, Tew JM Jr. Transpetrosal approach: surgical anatomy and technique. Neurosurgery 1993;33:461–469, discussion 469 PubMed
17. Sen CN, Sekhar LN. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery 1990;27:197– 204 PubMed
18. Nanda A, Vincent DA, Vannemreddy PS, Baskaya MK, Chanda A. Far-lateral approach to intradural lesions of the foramen magnum without resection of the occipital condyle. J Neurosurg 2002;96:302–309 PubMed
19. Peerless SJ, Drake CG. Management of aneurysms of posterior circulation. In: Youmans JR, ed. Neurological Surgery. A Comprehensive Guide to the Diagnosis and Management of Neurosurgical Problems, Vol 3. Philadelphia: WB Saunders, 1982:1715–1763
20. Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg 1997;87: 141–162 PubMed
21. Peerless SJ, Hernesniemi JA, Gutman FB, Drake CG. Early surgery for ruptured vertebrobasilar aneurysms. J Neurosurg 1994;80:643–649 PubMed
22. Heros RC. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg 1986;64:559–562 PubMed
23. Salas E, Sekhar LN, Ziyal IM, Caputy AJ, Wright DC. Variations of the extreme-lateral craniocervical approach: anatomical study and clinical analysis of 69 patients. J Neurosurg 1999;90(2, Suppl):206–219 PubMed
24. D’Ambrosio AL, Kreiter KT, Bush CA, et al. Far lateral suboccipital approach for the treatment of proximal posteroinferior cerebellar artery aneurysms: surgical results and long-term outcome. Neurosurgery 2004;55:39–50, discussion 50–54 PubMed
25. Bertalanffy H, Seeger W. The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery 1991;29:815–821 PubMed
26. Sekhar LN, Kalia KK, Yonas H, Wright DC, Ching H. Cranial base approaches to intracranial aneurysms in the subarachnoid space. Neurosurgery 1994;35:472–481, discussion 481–483 PubMed
27. Samii M, Klekamp J, Carvalho G. Surgical results for meningiomas of the craniocervical junction. Neurosurgery 1996;39:1086–1094, discussion 1094–1095 PubMed
28. al-Mefty O, Borba LA, Aoki N, Angtuaco E, Pait TG. The transcondylar approach to extradural nonneoplastic lesions of the craniovertebral junction. J Neurosurg 1996;84:1–6 PubMed
29. Heros RC. Stroke: early pathophysiology and treatment. Summary of the Fifth Annual Decade of the Brain Symposium. Stroke 1994;25:1877– 1881 PubMed
30. Kumar CR, Vannemreddy P, Nanda A. Far-lateral approach for lower basilar artery aneurysms. Skull Base 2009;19:141–149 PubMed
31. Sekhar LN, Kalia KK, Yonas H, Wright DC, Ching H. Cranial base approaches to intracranial aneurysms in the subarachnoid space. Neurosurgery 1994;35:472–481, discussion 481–483 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








