CHAPTER 300 Anterior Thoracic Instrumentation
Historical Background
The impetus for developing thoracic instrumentation can be found in the literature as far back as 1928, when Royle described anterior decompression of the spine for scoliotic deformities.1 Hodgson and Stock later described similar decompressive procedures for Pott’s disease.2 These efforts did not include spinal reconstruction; as a result, their patients suffered from postoperative instability and progressive deformity. In 1958, Humphries and Hawk published one of the first reports on ventral instrumentation developed for stabilization after transperitoneal débridement in a patient with Pott’s disease.3 Their ventral plate and screw construct provided little biomechanical support and was later abandoned. In the 1970s, advances in construct design were introduced by Dwyer,4,5 Zielke,6 and colleagues. The screw-cable construct of Dwyer and the screw-rod construct of Zielke successfully corrected scoliotic deformities. However, these constructs were not rigid enough to provide structural support in the setting of significant spinal instability. In the late 1970s, Dunn developed a double-rod, double-screw construct that provided adequate stability for anterior thoracolumbar reconstruction.7,8 In 1986, however, the Dunn device was removed from clinical use after reports of great vessel erosion.9 Since the Dunn device, improved material properties and implant designs have led to lower profile constructs, which essentially eliminated the risk for long-term vessel erosion, as well as magnetic resonance imaging–compatible implants. Today’s spine surgeon has numerous options to effectively stabilize the anterior thoracic spine.
Indications for Operative Intervention
Neoplasm
Most neoplastic disorders affecting the spinal column are malignant. Spinal neoplasms can be manifested as pain, progressive deformity, or neurological deficits. Operative intervention is appropriate for tissue diagnosis, neurological decompression, and spinal stabilization. Other indications include resection of radioresistant neoplasms, resection of isolated recurrences, and neurological deterioration after adjuvant treatment. Less optimal outcomes with an increased incidence of postoperative complications have traditionally been associated with posterior decompression for anterior metastatic disease.10 Clinical outcomes were so poor that radiotherapy became the treatment of choice.10–14
Advances in surgical technique led to the development of ventral spinal approaches without excessive complications. An anterior approach provides a direct means of addressing ventral pathology and reconstructing the spine at the primary site of instability.10 Currently, clinical outcomes in patients with metastatic spinal disease after anterior decompression are superior to those after posterior decompression with or without adjuvant radiotherapy.15–22 However, selection of appropriate patients for surgical intervention remains a challenge.
Important considerations for appropriate patient selection include age, preoperative functional status, presence of medical comorbid conditions, life expectancy, and need for tissue diagnosis.23–25 Typically, surgical intervention is reserved for patients with a life expectancy of at least 6 to 12 months; however, surgery should not be withheld if it will significantly improve quality of life. The value of restoring neurological function, such as ambulation or sphincter control, must be judged on an individual basis. Other considerations in selecting patients for operative intervention include the type of tumor, compromise of the spinal canal, extent of neurological deficits, level of pain, and degree of instability. Adjuvant treatment such as radiotherapy, chemotherapy, or hormonal therapy may be indicated in specific cases. Additionally, nonoperative intervention remains an option for patients with stable myelopathy despite spinal cord compression.11,26,27 The surgeon must take all these factors into consideration on a case-by-case basis.
Neurological deterioration is rare after operative intervention for metastatic spinal disease. Perioperative mortality rates range from 6% to 8%, and overall complication rates range from 8% to 11%.15 In these reports, some patients were not treated initially with an anterior approach, and it is possible that an anterior approach alone would have decreased the combined rate of morbidity and mortality.15
Trauma
Most thoracic spine fractures occur at the thoracolumbar junction; about 50% to 80% occur between T10 and L2.28,29 The thoracolumbar region is particularly susceptible to traumatic injury for several reasons: instability associated with transition from the stable thoracic spine to the mobile lumbar region, loss of rigidity provided by the intact rib cage, instability associated with transition from kyphosis to lordosis, and change in orientation of the facet joints.30,31 Based on the three-column model of stability, Denis categorized these fractures into four classes: compression fractures, burst fractures, seat belt–type fractures, and fracture-dislocations.32 The most common types of fractures are compression and burst fractures.32 Traditionally, fractures that produce a 40% loss in height of the vertebral body, a 50% compromise of the spinal canal without neurological deficits, a kyphotic deformity of 30 degrees, or neurological deficits require operative intervention.33,34
The choice of surgical procedure remains controversial. Posterior fusion and fixation may be appropriate in the absence of a ventral deformity or anterior spinal canal compromise. Lesions producing ventral compression or significant biomechanical compromise of the anterior and middle columns are indications for an anterior approach. Previous reports have documented better outcomes with an anterior approach than with isolated posterior stabilization.33,35–37 If the posterior elements have been compromised, however, an isolated anterior construct may be insufficient to resist flexion forces. Loss of the posterior tension band may require supplementation with a posterior stabilization construct. Patients with a complete spinal cord injury may still benefit from spinal stabilization. In such circumstances, surgical stabilization optimizes the rehabilitation process and possibly decreases the length of hospitalization.33–35,38–41
Infection
Hodgson and Stock were among the first to describe successful anterior decompression in patients with Pott’s disease.2 With advances in diagnosis and antibiotics, most cases of vertebral osteomyelitis and diskitis can now be treated effectively without surgical intervention. As many as 90% of patients respond to intravenous antibiotics and immobilization.42 Surgical débridement and stabilization are necessary when the infection is resistant to intravenous antibiotics, the causative organisms cannot be identified, neurological deficits progress in the presence of a ventral collection, and severe osseous destruction leads to a significant deformity or intractable pain.
Historically, there has been concern regarding the insertion of instrumentation into an infected spine; however, clinical series have demonstrated that the presence of an active infection does not appear to be a contraindication to insertion of spinal implants,43–45 and in fact, anterior instrumentation has been shown to be safe for the treatment of patients with pyogenic vertebral osteomyelitis of the thoracic and lumbar spine after anterior débridement and autogenous bone grafting.46 In addition, it has also been shown to provide segmental stability, correction of kyphotic deformity, and promotion of bony fusion in patients with spinal tuberculosis.47
Deformity and Degenerative Conditions
Symptomatic deformity of the thoracic spine can occur as a late complication of trauma, infection, neoplasm, or previous surgery. Surgery is often reserved for kyphosis greater than 30 degrees, as well as radiographic evidence of progression of the deformity. Clinical indications for operative intervention include intractable axial or radicular pain and progressive neurological deficits. At the thoracolumbar junction, instrumentation is necessary to resist the dynamic biomechanical forces encountered at this level.48–51
In the spine, the overwhelming majority of degenerative disease is encountered rostral or caudal to the thoracic region. Disk herniations are the most common type of degeneration at the thoracic level. Most of these rare lesions can be resected successfully through a posterolateral approach without the addition of fusion and fixation.52
Surgical Approaches to the Anterior Thoracic Spine
Approach to the Upper Thoracic Spine
Depending on the patient’s body habitus, exposure of the first or second thoracic vertebrae can occasionally be accomplished through a traditional anterior cervical approach. A more extensive sternal-splitting approach is usually necessary for anterior access to the lower vertebrae, typically up to the fourth or fifth thoracic vertebrae, or for patients with short stout necks. These exposures should be performed with the assistance of an experienced thoracic surgeon and are beyond the scope of this text.53–57 Numerous references are available that describe these approaches in more detail.55,58–61
Lateral Extracavitary Approach
The lateral extracavitary approach introduced by Larson and colleagues62 in 1976 is a derivative of the lateral costotransversectomy. It is ideally suited for lesions with a significant anterior paraspinal extension, with or without a substantial intraspinal component. This approach provides a familiar exposure and orientation for neurosurgeons and extensive access to all three spinal compartments.63 The lateral extracavitary approach is entirely extrapleural and avoids the perioperative complications associated with transthoracic exposure. In addition, circumferential stabilization of the spine is possible through a single incision. The lateral extracavitary approach is versatile and can be modified according to the size of the tumor, level of involvement, relationship to neural structures, and need for spinal stabilization.
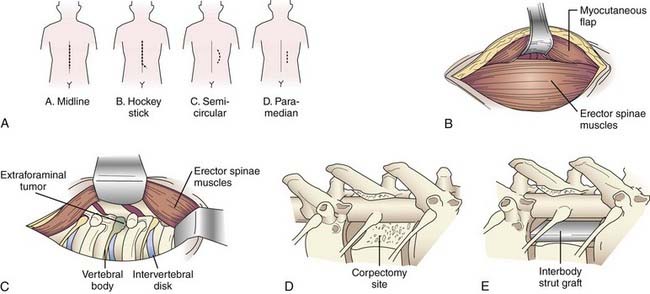
A variety of incisions can provide access via the lateral extracavitary approach, including midline, hockey stick, semicircular, and paramedian incisions (Fig. 300-1A). If a “hockey stick” type of incision is used, adequate caudal exposure is needed before the incision is extended laterally, or the caudal extent of the exposure will be limited. Routine subperiosteal dissection of the paraspinal muscles is performed with a Cobb dissector and monopolar cauterization. The takedown is performed bilaterally if posterior exposure is required for laminectomy or instrumentation; otherwise, unilateral exposure is sufficient. The lateral limb of the “hockey stick” incision is opened by transecting the transversely oriented thoracic muscles (i.e., latissimus dorsi, trapezius, rhomboid) in line with the skin incision. The myocutaneous flap is elevated to expose the longitudinally oriented erector spinae musculature (Fig. 300-1B). An extended midline incision can provide similar access with less musculoligamentous injury and potentially an improved cosmetic result.
Once the transversely oriented muscles are retracted, the lateral margin of the erector spinae is identified and dissected medially to expose the underlying ribs. This dissection is then carried over the facet joint and eventually communicates with the posterior dissection. Mobilization of the erector spinae muscle allows access to the lateral spinal compartment (Fig. 300-1C). The longitudinal muscle mass is wrapped in a moist sponge to avoid desiccation and positioned medially or laterally to allow the surgeon to work on either side.
Once the rib and transverse processes are removed, the neurovascular bundle is identified within the endothoracic fascia, deep to the intercostal musculature. The segmental nerves above and at the level of the pathology are dissected free and divided, with the proximal stump elevated medially toward the intervertebral foramen. Unlike the situation at the lumbar levels, nerves at the thoracic levels can be sacrificed without incurring a functional deficit. Once the nerve is free, the parietal pleura is displaced ventrally to provide access to the anterolateral spinal compartment. The parietal pleura is kept out of the operative field with a table-mounted retractor system. A padded, wide retractor blade helps avoid a pleural tear. The parietal pleura, diaphragm, or both are bluntly dissected free from the ventrolateral aspect of the vertebral bodies with a Cobb elevator. Sharp dissection may be required at the level of the disk space if more adherent connective tissue is present. The sympathetic chain is identified along the ventrolateral aspect of the vertebral bodies. The rami communicantes are transected at the involved spinal segments, and the sympathetic chain is mobilized by subperiosteal dissection. The dorsal and foraminal vessels are identified, cauterized, and transected. The previously elevated nerve stump facilitates dissection to the foramen and pedicle. Resection of the pedicle allows identification of the ventral spinal canal. A distinct advantage of the lateral extracavitary approach is the simultaneous access provided to both the anterolateral and the posterior spinal compartments. The spinal canal can be decompressed by resection of both the posterior and anterior elements (Fig. 300-1D). Once the pathology has been resected, an interbody spacer can be placed (Fig. 300-1E) and circumferential spinal stabilization can be performed. The parietal pleura is inspected for air leaks. Minor leaks can be repaired primarily. A significant leak mandates placement of a chest tube during the immediate postoperative period. Multilayer wound closure is performed while making sure to reattach the appropriate muscle layers.
Retropleural Thoracotomy
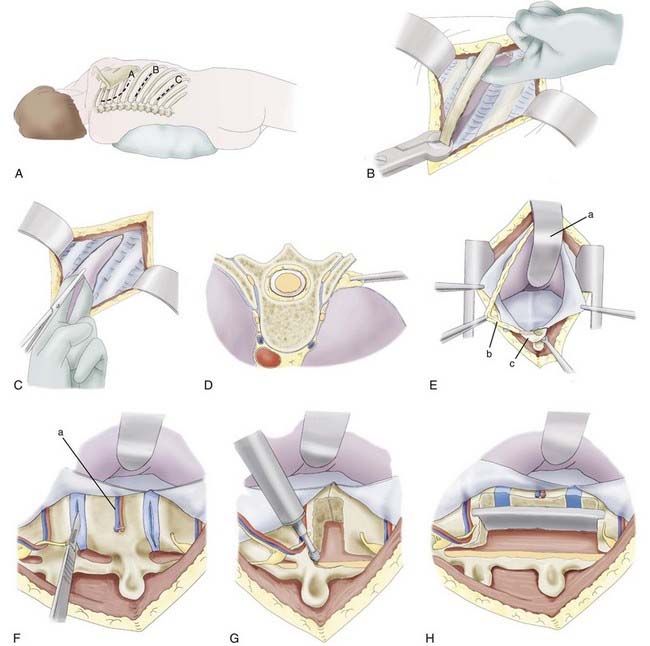
The patient is placed in a lateral position with an axillary roll and appropriate padding to prevent pressure neuropathies. Because of the caudal rib angulation, the incision for an anterolateral approach should be made two levels above the pathology. For example, a lesion at T8 would be approached through an incision over the sixth rib. The incision for a retropleural approach starts approximately 4 cm from the midline over the rib of interest and extends to the midaxillary line (Fig. 300-2A). The subcutaneous tissue and underlying muscles are transected with monopolar cauterization to expose the periosteum of the rib. The surrounding soft tissue is detached from the dorsal aspect of the rib with an Addison dissector while preserving the intercostal neurovascular bundle. A Doyen dissector is used to free the ventral periosteum along the length of the rib. The dissection continues as far medially as possible, usually 1 to 2 cm lateral to the costotransverse joint. The section of rib is resected and saved for possible grafting (Fig. 300-2B).
The endothoracic fascia is identified as the shiny tissue layer deep to the rib periosteum but superficial to the parietal pleura. This fascial layer is sharply incised in line with the rib bed (Fig. 300-2C). The underlying parietal pleura is freed in all directions by blunt dissection with a finger or sponge dissector (Fig. 300-2D). This maneuver exposes the costotransverse joint and anterolateral aspect of the vertebral bodies. For exposure of the lower thoracic region and thoracolumbar junction, the lateral attachments of the diaphragm must be divided so that the retropleural and retroperitoneal spaces are in communication. Instead of incising the diaphragm circumferentially from the anterior chest wall, the 11th and 12th rib attachments to the diaphragm are dissected subperiosteally through a smaller, more caudal diaphragmatic opening. The dissection continues medially to elevate the lateral and medial arcuate ligaments from the underlying muscles. At the transverse process of L1, a cuff of muscle is left intact to help reapproximate the diaphragm. Finally, the left crus is divided to complete the communication of the retropleural and retroperitoneal cavities. The peritoneum is gently swept from the posterior abdominal wall, with special attention paid to the area near the central tendon, where the peritoneum is thinner. The hemiazygos and accessory azygos veins should be identified and may require cauterization or clipping. The proximal rib head is resected sharply to expose the lateral aspect of the vertebral body (Fig. 300-2E). A table-mounted retractor is positioned and used to retract the lung and peritoneal contents. Use of a well-padded retractor blade avoids injury to the underlying structures. Once stabilization is complete, multilayer closure is performed.
Corpectomy and Fusion
After adequate exposure is achieved, the corpectomy is performed. Before the vertebral body is resected, the rostral and caudal intervertebral disks are removed. The lateral surface of the disk is incised, and the disk material is extracted with pituitary rongeurs and curets (Fig. 300-2F). After removal of the disk, the pedicle and facet joint are removed with either a high-speed drill or a rongeur. During these maneuvers, the epidural space is probed with a nerve hook or blunt dissector to ensure that the thecal sac and nerve roots are freed. While drilling, adequate irrigation is necessary to avoid thermal injury to the underlying nerve root and spinal cord.
Ventral decompression is achieved by first drilling the anterior aspect of the vertebral body to create a ventral defect (Fig. 300-2G). With a reverse-angle curet, the remaining dorsal bone is delivered into the trough created, and the corpectomy is completed. Considerable blood loss is possible because of the proximity of the perineural venous plexus. The anesthesiologist should be advised of this possibility so that excessive blood loss can be replaced adequately. A lesion within the ventral spinal canal can be delivered into the corpectomy site with the use of a reverse-angle or down-angled curet. Contralateral visualization of the spinal canal can be obtained with an angled dental mirror.
The height of the corpectomy defect and width of the rostral and caudal end plates are measured to determine the appropriate graft size. Many devices are now equipped with templates and calipers to make these calculations more precise. The graft is cut or constructed to the appropriate size, and a trial insertion is performed. Ideally, a nonexpandable graft should be slightly longer than the relaxed corpectomy site so that retraction of the distracted ligaments will hold the graft in place. The vertebral bodies are distracted, and the graft is inserted with a mallet and impactor. The graft can be mortised into the end plates to help prevent dislodgment of the graft (Fig. 300-2H). Alternatively, an expandable cage can be inserted in the reduced configuration and, according to manufacturer guidelines, expanded to engage the end plates. Excessive distraction of the expandable cage should be avoided because the expansion mechanism typically provides the surgeon with a biomechanical advantage that may not be appreciated during insertion; several reports imply that this biomechanical advantage leads to an increased incidence of graft subsidence.90,91,93 After placement, the graft should lie flush with or slightly recessed from the ventral edges of the vertebral bodies. Corticocancellous bone chips are placed ventral to the graft to enhance the potential for fusion. The posterior border is checked with a nerve hook to ensure that the graft does not impinge on the spinal cord.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree