FIGURE 11.1 Diagram of the path of the pupillary light reflex. (From Miller NR, Newman NJ, Biousse V, et al, eds. In: Walsh and Hoyt’s Clinical Neuro-Ophthalmology: The Essentials. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008:316.)
Pupillary dilation is a reflex response to sudden arousal or darkness and is mediated by the sympathetic pathway (Fig. 11.2). The oculo-sympathetic pathway is a three-neuron pathway that originates in the hypothalamus and signals contraction of the iris dilator muscle.
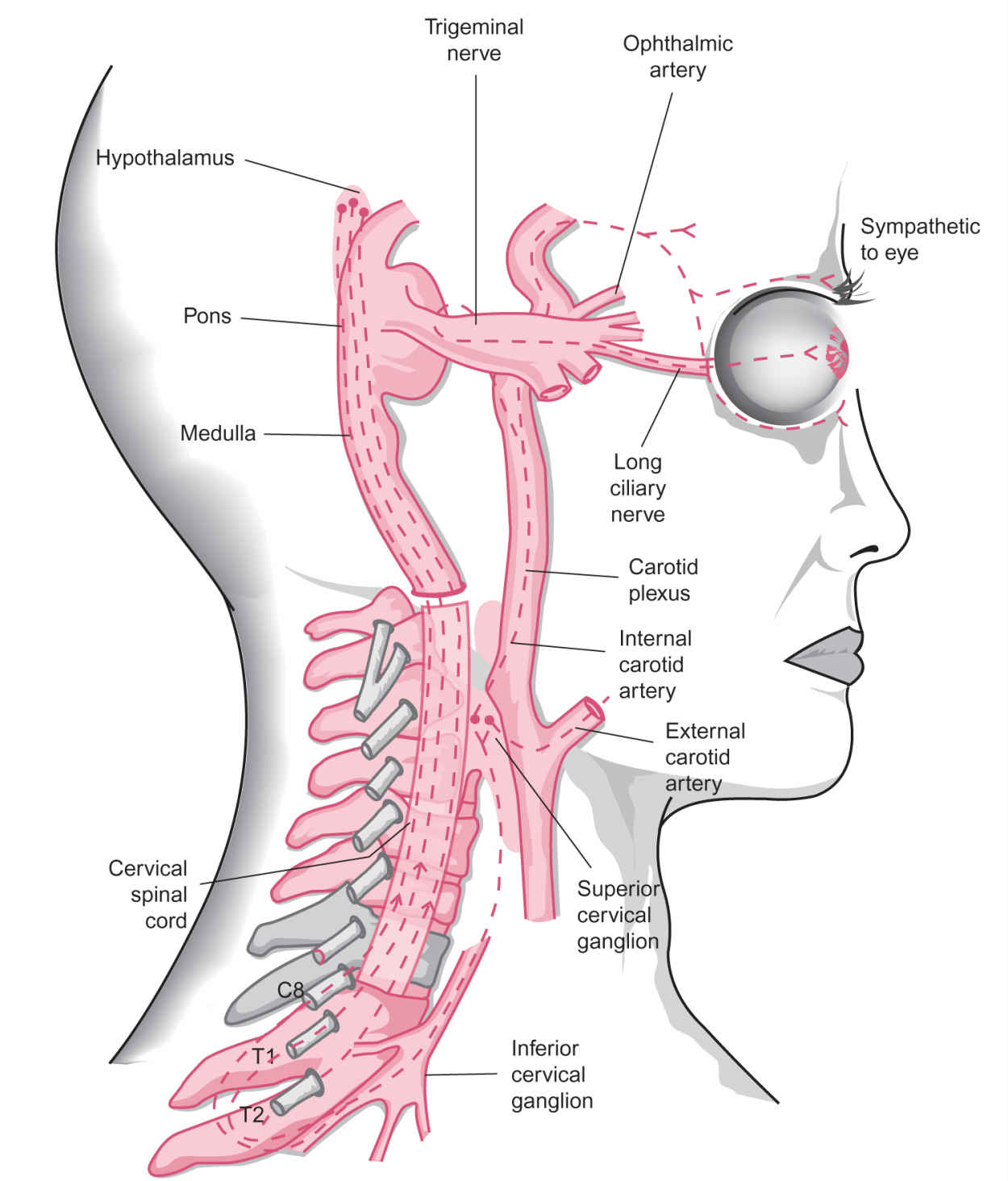
FIGURE 11.2 Sympathetic innervation of the pupil and eyelids. First-order hypothalamic (central) neurons descend through the brain stem (midbrain, pons, medulla) and cervical spinal cord. These fibers then synapse with preganglionic neurons whose cell bodies like in the intermediolateral gray column and whose axons exit the core ipsilaterally at C8; T1 and T2 via the ventral roots. These second-order (preganglionic) fibers travel rostrally via the sympathetic chain, traverse the superior mediastinum, pass through the stellate ganglion (the fusion of the inferior cervical ganglion and the first thoracic ganglion), and terminate in the superior cervical ganglion, which lies posterior to the angle of the mandible. The postganglionic axons ascend within the carotid plexus, which surrounds the internal carotid artery to reach the cavernous sinus. The pupil fibers briefly join the sixth nerve then follow branches of the first division of the trigeminal nerve and the long ciliary nerve to reach the iris dilator muscle. Fibers to the tarsal muscles (Müller’s muscles) also travel within the carotid plexus to the cavernous sinus then may join branches of the third nerve before reaching the upper and lower eyelids. Sudomotor fibers, for example, for sweating to the lower face, follow the external carotid and then the facial arteries. (From Liu GT, Volpe NJ, Galetta SL, eds. Neuro-Ophthalmology Diagnosis and Management. 2nd ed. Philadelphia, PA: WB Saunders/Elsevier; 2010. Reprinted with permission.)
Both the static and dynamic aspects of the pupils should be examined. This includes noting the resting pupil size in dim and bright illumination, comparing the speed and amplitude of pupillary movement to a light stimulus (and to darkness, if indicated) and comparing the pupil response between the two eyes.
A. Hippus. An awake patient sitting quietly in room light may show small, irregular, spontaneous oscillations of pupil size, known as hippus. It reflects fluctuations in the central modulating signals to the Edinger–Westphal subnuclei.
B. Pupil size
Baseline pupil size is dependent on many factors, and in particular the level of ambient light. Under dim light conditions, pupils are largest (7 to 9 mm) during the teenage years and then gradually decrease with increasing age. Asymmetry of pupil size, or anisocoria, of 0.4 mm or more is visible to the unaided eye. Depending on the pathology, the magnitude of anisocoria varies under different light conditions.
1. Anisocoria whose magnitude is greater under bright light suggests an inability of the larger pupil to constrict. This may be due to a defective iris, sphincter muscle failure, or impairment along the oculo-parasympathetic pathway.
2. Anisocoria that is more apparent in darkness implies a failure of dilation; thus, the smaller pupil is likely to be the faulty one.
C. Testing the pupil light reflex
Have the patient fixate on a distant target in a darkened room. Shine a bright focal light (a non-halogen penlight is not bright enough) directly onto one pupil for 3 seconds and note the speed and amplitude of constriction. Do this two or three times for a mental “average.” Remember that a pupil that constricts poorly to direct light stimulation may be due to an optic neuropathy (defective afferent limb), interruption along the oculo-sympathetic pathway (defective efferent limb), or a damaged iris sphincter (defective mechanics). The latter two mechanisms result in anisocoria.
D. Performing the alternating light test
This is the standard clinical technique for identifying asymmetry of afferent pupillomotor input between the two eyes, referred to as the relative afferent pupillary defect (RAPD).
1. Technique. Have the patient fixate a distant target in a dark room. Shine a bright focal light directly onto one pupil for 3 seconds, and then quickly swing the light onto the other pupil for 3 seconds. Repeat this for four or five alternations of light stimulation and watch the illuminated pupil (direct light response). The amplitude and velocity of pupillary constriction as well as the degree of redilation that occurs within the 3 seconds of light stimulation should be symmetric between the two eyes.
2. A large RAPD is present if the pupil of the “bad” eye simply dilates when the light is alternated back onto it after being on the “good” eye. In other words, the bad eye sees so little, if any, light that reflex dilation to darkness is instead initiated. Note that an RAPD is not a cause of anisocoria (Video![]() 11.1).
11.1).
3. A small-to-moderate RAPD is sometimes difficult to detect. The bad eye “sees” enough light to initiate pupillary constriction on direct stimulation but it is a less vigorous response compared to that of the good eye. The pupil of the bad eye also “escapes,” that is, redilates sooner after the initial constriction (Video![]() 11.2).
11.2).
RELATIVE AFFERENT PUPILLARY DEFECT
The presence of an RAPD indicates unilateral or asymmetric injury to the afferent limb of the pupillary light reflex. In most instances, an RAPD is associated with optic nerve damage. Cataracts, corneal opacities, and vitreous lesions do not cause an RAPD, despite causing significant visual disturbance.
A. Optic nerve lesions.
1. An RAPD is a sensitive indicator of optic nerve dysfunction. A small RAPD often persists in patients with previous optic neuritis in whom vision has recovered.
2. The magnitude of RAPD is better correlated with the extent of visual field loss than with visual acuity. Patients with optic neuropathy and RAPD can have 20/20 acuity.
3. The extent of damage in bilateral optic nerve disorders is rarely symmetric. Therefore, an RAPD is generally found on the side with greater damage. An exception to the rule occurs in patients with Leber hereditary optic neuropathy or autosomal-dominant optic atrophy. These patients have visual loss because of optic nerve dysfunction and yet maintain relative preservation of the pupil light reflex. This visual–pupillary dissociation is presumably related to a greater resistance of intrinsically photosensitive retinal ganglion cells to neurodegeneration caused by mitochondrial dysfunction.
B. Retinal lesions. Less commonly, an RAPD results from severe retinal disorders associated with severe visual loss.
1. Large unilateral retinal lesions such as central retinal artery occlusion, large retinal detachment, or trauma produce an obvious RAPD. A dilated funduscopic examination usually confirms the diagnosis.
2. In the patient with poor acuity, a central scotoma and yet displays no-to-minimal RAPD, look for a macular lesion. Multifocal electroretinography may be indicated.
C. Optic chiasm. Lesions of the optic chiasm that produce bilateral but asymmetric visual dysfunction cause an RAPD in the eye with greater amount of visual field loss.
D. Optic tract lesions. In the patient with a homonymous hemianopsia due to an optic tract lesion, an RAPD may be present in the contralateral eye. A tract RAPD reflects the greater proportion of decussating axons compared to nondecussating axons at the optic chiasm.
E. Pretectal nucleus. A unilateral dorsal midbrain lesion such as stroke or tumor that damages the pretectal olivary nucleus on one side can produce a small RAPD in the contralateral eye. This is the rare occurrence of RAPD without associated visual loss.
MECHANICAL ANISOCORIA: “OPHTHALMOLOGIC” ANISOCORIA
Two iris muscles modulate pupil size and shape—the sphincter and the dilator. Damage to one or both iris muscles can distort the size, shape, and mobility of the pupil. Ocular pathologies such as trauma, infection, or surgery are common causes of a mechanical anisocoria. It is important to consider and identify ocular causes of anisocoria in order to avoid unnecessary neurologic evaluation.
A. History. Inquire about any previous infection, inflammation, trauma, or surgery involving the eyes, including laser procedures.
B. Examination
1. Marked irregularity of the pupillary margin, unusual distortion of pupillary shape, and a difference in iris color suggest that the iris structure and framework are damaged. Such findings are appreciated with direct observation (Fig. 11.3).
2. A slit lamp examination of the iris is needed to identify most other causes of mechanical anisocoria such as synechiae (adhesions), small sphincter tears, transillumination defects, and inflammation (Fig. 11.3). A dusting of iris pigment may form a ring on the lens of a patient who has had a blow to the eye.
UNILATERAL MYDRIASIS: NEUROLOGIC OR PHARMACOLOGIC?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







