A. Otologic vertigo is caused by dysfunction of the inner ear. Table 16.1 lists entities that account for about 95% of all cases of otologic vertigo. The distribution of diagnoses varies greatly according to the referral base (e.g., neurology, otolaryngology, general medicine, emergency room), but in all settings otologic vertigo comprises a substantial component.
1. Benign paroxysmal positional vertigo (BPPV) has an incidence of about 0.6% per year, making it the most common otologic cause of vertigo (nearly 50%) and accounting for roughly 20% of all cases of vertigo, thus also making it the single most common cause of vertigo over the life span. BPPV presents with brief vertigo provoked by changes in the orientation of the head with respect to gravity. BPPV is caused by loose debris within the labyrinth.
2. Vestibular neuritis presents with vertigo, nausea, ataxia, and nystagmus. Limited evidence implicates a viral infection of the vestibular nerve as the etiology. Labyrinthitis presents with the same vestibular symptom complex, combined with aural symptoms of tinnitus and/or hearing loss. Vestibular neuritis and labyrinthitis together account for about 15% of all otologic vertigo cases.
3. Ménière’s disease presents with intermittent vertigo accompanied by hearing complaints (see the so-called “hydrops” symptom complex in Section A.3 under Differential Diagnosis). It accounts for about 15% of otologic vertigo cases.
4. Bilateral vestibular paresis presents with oscillopsia and ataxia, usually caused by loss of vestibular hair cells. The typical history is of treatment for several weeks with an intravenous or intraperitoneal ototoxic antibiotic (of which gentamicin is the most commonly used). Bilateral vestibular loss is uncommon.
5. The superior semicircular canal dehiscence (SSCD) syndrome exemplifies several conditions in which there is an abnormal opening between the inner ear and a surrounding structure. They generally present with vertigo induced by sound (called Tullio’s phenomenon) or as ataxia provoked by activity or straining. The diagnosis of SSCD has been rapidly increasing in recent years because of a combination of improved knowledge about the condition combined with greater use of a new diagnostic modality—vestibular evoked myogenic potentials (VEMPs), and thin-section computed tomography (CT) protocols providing detailed imaging coplanar with the superior semicircular canal. In SSCD, bone over the superior semicircular canal is absent. Similar symptoms are seen in perilymphatic fistula and cholesteatoma.
6. Tumors compressing the eighth cranial nerve, such as acoustic neuroma, present with asymmetric hearing loss combined with mild ataxia. Eighth-nerve tumors are very uncommon in the vertiginous population (but are more common in the unilaterally hearing impaired).
B. Central vertigo is caused by dysfunction of central structures that process sensory input from the inner ear. Central vertigo accounts for 2% to 50% of vertigo diagnoses, depending on the setting in which patients are seen. In a majority of cases, central vertigo is caused by migraine. Table 16.1 lists entities accounting for about 90% of central vertigo diagnoses, the remainder being made up of individual unusual conditions (e.g., spinocerebellar degeneration).
1. Migraine-associated vertigo (also called “vertiginous migraine,” “vestibular migraine,” and similar labels) ordinarily presents with vertigo and headache, but it can also present as isolated vertigo. Migraine causes about 75% of central vertigo cases, and is the most common cause of vertigo in the pediatric population. It is particularly common in women in their 30s and 50s.
2. Stroke and transient ischemic attack (TIA) involving the brainstem or cerebellum is an occasional source of central dizziness. Vertigo can occasionally be the only symptom preceding a posterior fossa stroke; there are not yet reliable means of distinguishing a TIA affecting the vestibular nucleus or cerebellum from another process affecting the vestibular nerve or end organ, although recent research on the head impulse test (HIT) suggests that this may be a promising approach to this diagnostic dilemma.
3. Seizures present with vertigo combined with motor symptoms or confusion. About 5% of central vertigo is caused by seizures. Dizziness is a common symptom in persons with known epilepsy.
4. Multiple sclerosis (MS) combines vertigo with other central signs such as cerebellar dysfunction. MS is an uncommon source of vertigo. About 2% of central vertigo cases are caused by MS. In persons with known MS, it is important not to attribute vertigo to MS without considering common peripheral causes that might be coincident, such as BPPV.
5. The Chiari malformation is a hindbrain malformation wherein the cerebellar tonsils herniate 5 mm or more below the foramen magnum. These patients complain of vertigo, ataxia, and occipital headaches, and often have downbeat nystagmus. Like SSCD, symptoms may be precipitated by straining. Magnetic resonance imaging (MRI) of the posterior fossa establishes the diagnosis. About 1% of cases of central vertigo are caused by the Chiari malformation.
6. Cervical vertigo is a controversial syndrome. Diagnosis is most often made after a whiplash injury where findings usually include vertigo, tinnitus, and neck pain. Examination usually demonstrates a nonspecific symptom complex including neck movement limited by pain and nausea or vertigo on neck positioning. Generally, there is no strong nystagmus even using video-recording methods. There are no definitive clinical or laboratory tests for cervical vertigo. MRI of the cervical spine in these patients often shows cervical disks abutting but not compressing the cervical cord. Rare cases have been reported in whom vertigo can be traced to compression of a vertebral artery after neck rotation (bow hunter syndrome). Due to the lack of clarity in the diagnosis of cervical vertigo, its prevalence is unknown.
C. Medical vertigo may be caused by altered blood pressure, low blood sugar, and/or metabolic derangements associated with medication or systemic infection. It is largely encountered in the emergency room, where it accounts for about 33% of all cases of dizziness. It is unusual in subspecialty settings (2% to 5%). Table 16.1 lists nearly all causes of dizziness reported in studies of vertigo as it presents to emergency rooms.
1. Postural hypotension often presents as giddiness, lightheadedness, or syncope. These symptoms occur only while the patient is upright.
2. Cardiac arrhythmia presents with syncope or drop-attacks. Like those of postural hypotension, symptoms are characteristically present only when patients are upright.
3. Hypoglycemia and metabolic derangements associated with diabetes present with giddiness or lightheadedness. Hypoglycemia is often accompanied by autonomic symptoms such as palpitations, sweating, tremors, or pallor. Together they account for about 5% of the cases of dizziness in general medical settings.
4. Medication effects usually present with giddiness or lightheadedness, but also can present with true vertigo. These diagnoses account for about 16% of the dizzy patients seen in the emergency setting, but are rare outside the emergency room. Medications commonly implicated include antihypertensive agents, especially α1-adrenergic blockers such as terazosin, calcium-channel blockers such as nifedipine, and sedatives. Benzodiazepines, such as alprazolam, can cause dizziness as part of the withdrawal syndrome. Alcohol intoxication can present as a transient positional nystagmus, cerebellar signs, and direction-changing positional nystagmus.
5. Viral syndromes not involving the ear are the reported cause of dizziness in approximately 4% to 40% of all cases seen in the emergency room setting. Such syndromes include gastroenteritis and influenza-like illnesses.
D. Unlocalized vertigo patients include those whose symptoms are attributed to psychiatric disorders, those whose symptoms are attributed to events without further definition (such as head trauma), and those with vertigo and dizziness of unknown origin. Common variants of unlocalized vertigo include psychogenic vertigo, hyperventilation syndrome, posttraumatic vertigo, and nonspecific dizziness. Between 15% and 50% of all patients with dizziness or vertigo fall into this category, depending both on referral base and diagnostic diligence.
1. Unknown (nonspecific dizziness). Diagnostic procedures are insensitive, and in dizziness evaluations it is usual to have as many as 50% of patients without any detectable abnormalities on careful clinical examination and thorough testing. Some authors wrongly define psychogenic vertigo as the complaints of patients falling into this category. About 75% of the unlocalized vertigo category consists of patients in whom there are no abnormalities on examination and testing.
2. Psychogenic. Patients with anxiety disorders, panic disorder, and posttraumatic stress disorder may complain of dizziness, ataxia, and autonomic symptoms. This is a common presentation. It is often impossible to determine whether or not anxiety is the sole cause or a reaction. In somatization disorder, symptoms may be present without anxiety.
3. Posttraumatic vertigo patients complain of vertigo following head injuries but frequently present no findings on examination or vestibular testing. BPPV is excluded by several negative Dix–Hallpike maneuvers using an adequately sensitive technique (e.g., video goggles). Posttraumatic vertigo is common. Diagnosis is sometimes complicated by medicolegal proceedings in which secondary gain must be taken into consideration.
4. Hyperventilation syndrome. These patients have vertigo after hyperventilation, without other findings or nystagmus. Hyperventilation-induced symptoms without substantial nystagmus are common in normal persons. Hyperventilation-induced symptoms are commonly seen in well-documented structural abnormalities such as acoustic neuroma.
5. Multisensory disequilibrium of the elderly. Most elderly people have age-related multisensory impairment. Like the diagnosis of psychogenic vertigo, this diagnosis is often used in situations where examination is otherwise normal.
6. Malingering. Because vertigo can be intermittent and disabling, and frequently follows head injury, it may be claimed in an attempt to obtain compensation. Malingering is common only among patients who are being compensated for illness.
CLINICAL MANIFESTATIONS
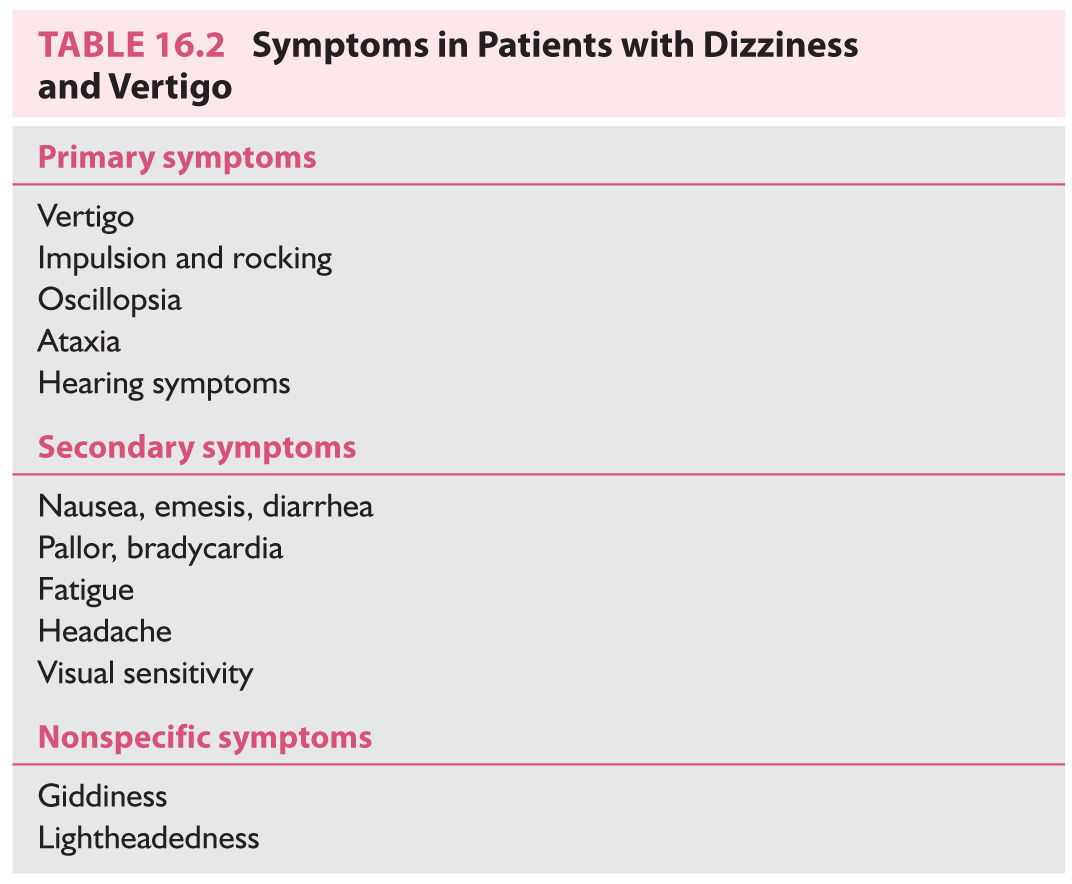
A. Primary symptoms. The primary symptoms listed in Table 16.2 are mainly the result of a disturbed sensorium.
1. Vertigo denotes a sensation of rotation—either of the person or of the world. It can be horizontal, vertical, or rotatory. It can be described as visual “blurring” or “jumping.” Horizontal vertigo is the most common type, usually resulting from dysfunction of the inner ear. Vertical vertigo is rarer. When transient, it is usually caused by BPPV. When constant, it is usually of central origin and accompanied by downbeat or upbeat nystagmus. Rotatory vertigo is the least frequent. When transient, rotatory vertigo is usually caused by BPPV. When chronic, it is always central and usually accompanied by rotatory nystagmus.
2. Impulsion denotes a sensation of translation, usually described as brief sensations of being pushed or tilted. Variants include rocking, floating, and perceived changes in the directions of up and down. Impulsion indicates dysfunction of the otolithic apparatus of the inner ear or central processing of otolithic signals. It is often a symptom of Ménière’s disease.
3. Oscillopsia is an illusory movement of the world evoked by head movement. Patients with bilateral vestibular loss are unable to see when their heads are in motion because of oscillopsia. Patients with unilateral vestibular loss often complain that “the world doesn’t keep up” or “my eyes lag behind my head” when they rapidly rotate their heads laterally to the side of the bad ear.
4. Ataxia, unsteadiness of gait, is nearly universal in patients with otologic or central vertigo and is variably observed in patients with medical and unlocalized vertigo.
5. Hearing symptoms. Vertigo is often accompanied by tinnitus, hearing reduction or distortion, and aural fullness.
B. Secondary symptoms include nausea, autonomic symptoms, fatigue, headache, and visual sensitivity. Visual sensitivity is also known as the “grocery store syndrome.” Patients complain of dizziness related to the types of patterned visual stimulation that occur when they traverse grocery store aisles, drive past picket fences or through bridges, or view large-screen movies. The grocery store syndrome is a nonspecific common late symptom in patients with vertigo and is generally thought to be caused by a reweighting of sensory input related to balance (ear, eye, and body) resulting in greater dependence on vision.
C. Giddiness, wooziness, heavy-headedness, and lightheadedness. These terms are imprecise although in common usage. They are rarely used by patients with documented inner ear dysfunction but are frequently used by patients with vertigo related to medical problems.
EVALUATION
A. History. The history must either be all-encompassing or follow a heuristic technique whereby questions are selected as the interview progresses. Here we outline the all-encompassing approach.
1. Definition. Does the patient complain of vertigo (spinning), a secondary symptom (such as nausea), a nonspecific symptom (giddiness or lightheadedness), or something entirely different (e.g., confusion)?
2. Timing. Are symptoms constant or episodic? If episodic, how long do they last?
3. Triggering or exacerbating factors are listed in Table 16.3. All patients should be queried regarding these factors, either by going through them one by one, or by using an interview heuristic whereby one attempts to rule in or rule out a symptom complex (see section Differential Diagnosis).
4. Otologic history. Ask about hearing loss, tinnitus, and fullness. Positives are indications for an audiogram. The patient’s description of tinnitus is sometimes helpful in diagnosis. For instance, “roaring” tinnitus often occurs in Ménière’s disease. “Buzzing” tinnitus sometimes occurs in vestibulocochlear paroxysmia. Pulsatile tinnitus suggests a vascular cause.
5. Medication history. Numerous medications can induce dizziness, including ototoxic drugs, antiepileptic drugs, antihypertensives, and sedatives. All current medications, as well as previous exposure to ototoxic agents, should be considered as sources of dizziness.
6. Family history. Has anyone in the immediate family had similar symptoms? Is there a family history of migraines, seizures, Ménière’s disease, or early-onset hearing loss? A family history of multiple relatives with conductive hearing loss suggests otosclerosis. The finding of hearing loss on the same side in multiple relatives suggests enlarged vestibular or cochlear aqueduct syndrome.
7. Review of systems should explore psychiatric problems (anxiety, depression, and panic), vascular risk factors, cancer, autoimmune disease, neurologic problems (migraine, stroke, TIA, seizures, and MS), otologic surgery, and general medical history (especially thyroid dysfunction, diabetes, Lyme disease, or syphilis).
8. Previous studies relevant to dizziness (see Section C under Evaluation) should be reviewed.
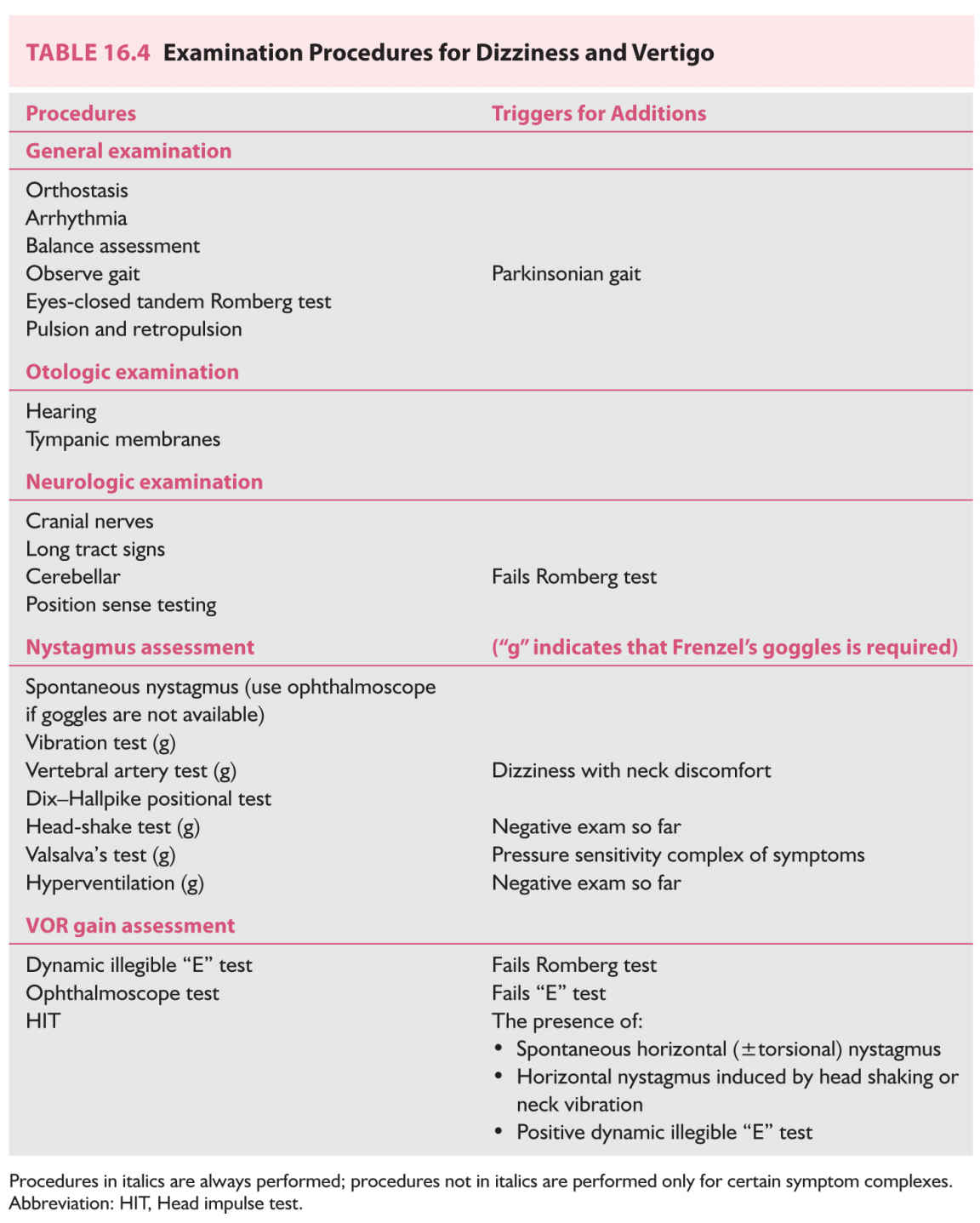
B. Physical examination. The physical examination of the vertiginous patient is outlined in Table 16.4. It is ordered in such a way that procedures may be added on the basis of previous results. Because a full examination may be lengthy, it is most practical to expand or contract the examination dynamically. As an exception to the following procedure, if there is a history of positional vertigo, it is reasonable to go immediately to the Dix–Hallpike test (see Section B.5.b under Evaluation).
1. General examination. Measure the blood pressure and pulse with the patient standing. Arrhythmia is noted, if present. If the standing blood pressure is low, check blood pressure with the patient lying flat. The heart and the carotid and subclavian arteries are auscultated. Besides, the mastoid is auscultated as well if there is pulsatile tinnitus.
2. Balance is assessed via observation of gait (see Chapter 8), and the eyes-closed tandem Romberg test. The tandem Romberg test is extremely useful. Low-normal performance consists of the ability to stand heel-to-toe, with eyes closed, for 6 seconds. Young adults should be able to perform this test for 30 seconds, but performance declines with age. It is helpful to develop a judgment of how much ataxia is appropriate for a given degree of ear injury. Patients with bilateral vestibular loss are moderately ataxic—they make heavy use of vision and are unsteady when their eyes are closed (with a narrow base). No patient with bilateral loss can stand in the eyes-closed tandem Romberg test for 6 seconds. Patients with an additional superimposed position sense deficit are unsteady with eyes open (with a narrow base). Patients with chronic unilateral vestibular loss show very little ataxia, and they are usually normal on the eyes-closed tandem Romberg test. The need to gauge ataxia does not come up in patients with recent unilateral vestibular imbalance because these patients have prominent nystagmus. Patients with cerebellar disorders, such as alcoholic cerebellar degeneration, have greater ataxia than is appropriate for their degree of nystagmus or vestibular paresis. Patients who are malingering also typically emphasize imbalance, which is the disabling aspect of their symptoms. In head injury or where there is other reason to suspect a central nervous system (CNS) origin of imbalance, also test basal ganglia function (pulsion/retropulsion tests).
3. Otologic examination. A brief screening test is adequate for hearing. The examiner’s thumb and first fingers are rubbed together at arm’s length from one of the patient’s ears. Persons with normal hearing can perceive this sound at an arm’s length. If the sound is not perceived, the source is brought in closer and closer until it is heard, and the distance is recorded. This simple test identifies high-tone hearing loss—for example, most elderly are able to hear at about 6 inches on either side. The tympanic membranes should be inspected for wax, perforation, otitis, discoloration, and mass lesions. Wax should be removed before more sophisticated diagnostic procedures such as audiograms or videonystagmograms are performed.
4. Neurologic examination. An abbreviated neurologic examination is adequate, though should be expanded appropriately if abnormalities are discovered. It is usually convenient to check the vestibulo-ocular reflex (VOR) and nystagmus with the ophthalmoscope at this point (see Sections B.5.a and B.6.b under Evaluation).
5. Nystagmus indicates an inner ear, brain, or (rarely) an ocular muscle disorder. Evaluation of nystagmus optimally requires use of Frenzel’s goggles, which are goggles worn by the patient to obscure their vision and magnify the examiner’s view of their eyes. Of the two Frenzel’s goggle variants available (optical and infrared video), the infrared video goggles are far superior. Without a method of viewing the eyes without fixation, almost all nystagmus procedures are either useless or very insensitive. If you use Frenzel’s goggles, mention this in your report. If you do not, indicate in your report that Frenzel’s goggles were not available.
a. Spontaneous nystagmus. With Frenzel’s goggles placed on the patient, the eyes are observed for spontaneous nystagmus for 10 seconds. The typical nystagmus produced by inner ear dysfunction is a primary position “jerk” nystagmus—the eyes slowly deviate off center and then there is a rapid “jerk,” which brings them back to the center position. Most nystagmus of other patterns (e.g., sinusoidal, gaze-evoked, saccadic) are of central origin. If Frenzel’s goggles are not available, similar information about spontaneous nystagmus can be obtained from the ophthalmoscopic exam. One simply monitors movement of the back of the eye. As the back of the eye moves oppositely to the front of the eye, for horizontal and vertical movement, one must remember to invert the direction of the nystagmus when making notes. Fixation can be removed by covering the opposite eye. Inner ear nystagmus is increased by removal of fixation. Congenital nystagmus is often reduced by removal of fixation.
b. Dix–Hallpike positional test (Fig. 16.1). The patient is positioned on the examination table so that, on lying flat, the head extends over the end of the table. The patient is then moved rapidly to this “head-hanging” position. If no dizziness or nystagmus is appreciated after 20 seconds, the patient is sat back up. The head is then repositioned to 45 degrees right, and the patient is brought down to the head-right supine position. After another 20 seconds, the patient is sat up again, and the procedure is repeated to the left (head-left supine position). One hopes to see a burst of nystagmus provoked by either the head-right or the head-left position. The nystagmus of the most common type of BPPV (posterior canal) beats upward and has a rotatory component, such that the top part of the eye beats toward the undermost ear ![]() (Video 16.1). The nystagmus typically has a latency of 2 to 5 seconds, lasts 5 to 60 seconds, and is followed by a downbeat nystagmus when the patient is sat up. There are also variant BPPVs with different vectors. The lateral-canal variant of BPPV is associated with a strong horizontal nystagmus that reverses direction between head left and right
(Video 16.1). The nystagmus typically has a latency of 2 to 5 seconds, lasts 5 to 60 seconds, and is followed by a downbeat nystagmus when the patient is sat up. There are also variant BPPVs with different vectors. The lateral-canal variant of BPPV is associated with a strong horizontal nystagmus that reverses direction between head left and right ![]() (Video 16.2). The anterior canal variant is associated with a downbeating nystagmus elicited by the Dix–Hallpike. The remainder of the nystagmus tests require video Frenzel’s goggles.
(Video 16.2). The anterior canal variant is associated with a downbeating nystagmus elicited by the Dix–Hallpike. The remainder of the nystagmus tests require video Frenzel’s goggles.
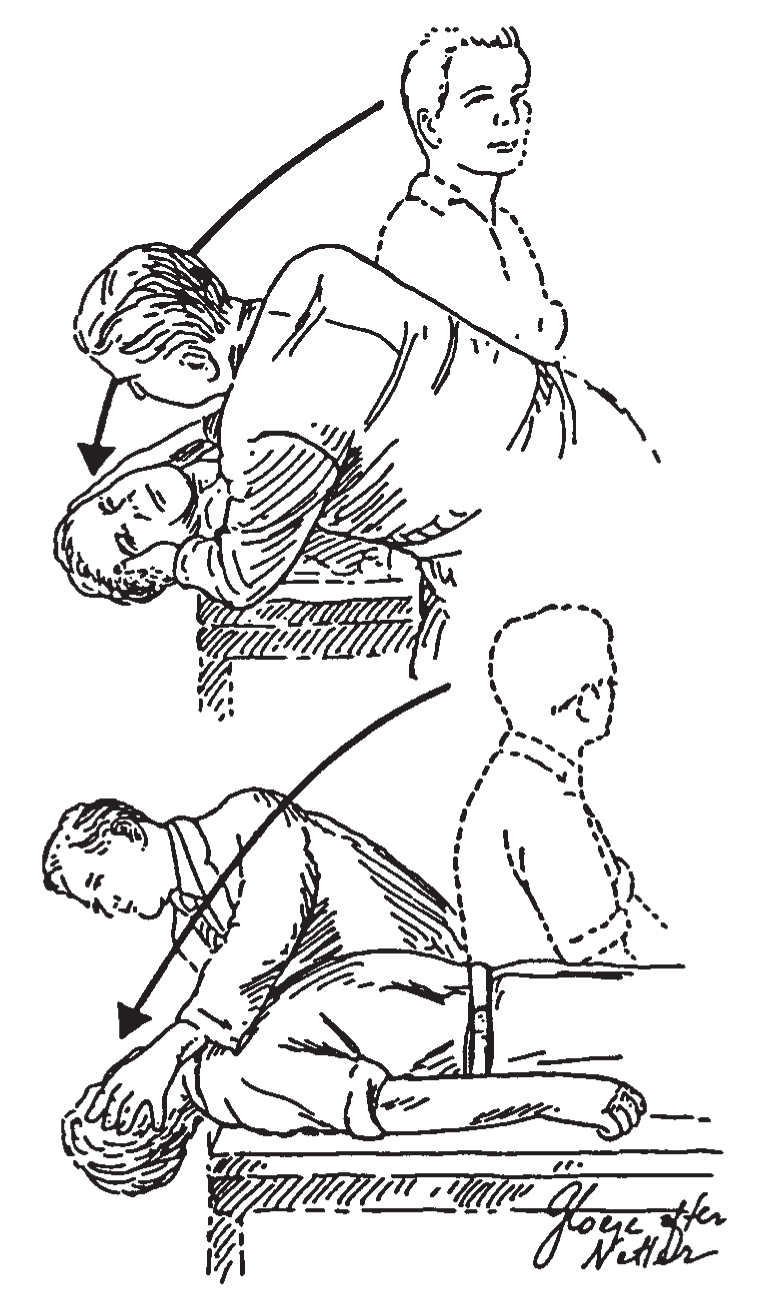
FIGURE 16.1 Dix–Hallpike positional test. To precipitate the characteristic nystagmus of BPPV, the patient is rapidly brought into a head position that makes the posterior canal vertical and also brings it through a large angular displacement. (From Baloh RW, Honrubia V. Clinical Neurophysiology of the Vestibular System. 2nd ed. Philadelphia, PA: FA Davis; 1990:124.)
c. Head-shake test. Performed if there is no spontaneous nystagmus or positional nystagmus. Wearing Frenzel’s goggles, the patient’s head is rotated by the examiner in the horizontal plane, back and forth, for 20 cycles. One aims for a 45 degree excursion of the head to either side and a frequency of 2 cycles per second. A nystagmus lasting 5 seconds or more is an indication of an organic disorder of the ear or CNS and supports further investigation.
d. Neck vibration test. The vibration test is more helpful than the head-shaking test. The eyes are observed in complete darkness while vibration (typically using a massager) is applied to the sternocleidomastoid for 10 seconds, first on one side and then on the other. A strong, direction-fixed nystagmus indicates a compensated peripheral vestibular lesion. The nystagmus beats away from the lesion.
e. Vertebral artery test for cervical vertigo. With the patient upright and wearing the goggles, the head is rotated to the end of rotation on either side and left there for 10 seconds. The eyes remain in the center. A positive test consists of a nystagmus provoked by the position of the head on trunk. Positives are very rare.
f. Valsalva’s test is performed if there is a pressure sensitivity symptom complex on history (see section Differential Diagnosis). While wearing the goggles, the patient takes a deep breath and strains for 10 seconds, while being observed for nystagmus. A positive test consists of nystagmus at the onset and release of pressure.
g. The hyperventilation test is performed if so far the examination has been entirely normal. The patient takes 30 deep, hard breaths. Immediately after hyperventilation, the eyes are inspected for nystagmus with Frenzel’s goggles and the patient is asked if the procedure has reproduced the symptoms. A positive test without nystagmus suggests the diagnosis of hyperventilation syndrome. Nystagmus induced by hyperventilation suggests a partially conducting eighth nerve or central vestibular pathways, such as due to a tumor of the eighth cranial nerve, gamma knife radiosurgery for acoustic neuroma, or MS.
6. Assessment of gain of the VOR. These maneuvers are aimed at documenting bilateral vestibular loss. They need not be done unless the patient has failed the eyes-closed tandem Romberg’s test.
a. The dynamic illegible “E” test. Using an eye chart at a distance of at least 10 feet, preferably calibrated in LogMar units, visual acuity is recorded with the head still. Then the examiner gently moves the patient’s head horizontally at roughly 1 Hz, ±30 degrees, and visual acuity is again recorded. Normal subjects drop from 0 to 2 lines of acuity with head movement. Patients with partial to complete bilateral loss of vestibular function drop from 3 to 7 lines of acuity. Patients with acute complete bilateral loss usually drop 7 lines of acuity.
b. The ophthalmoscope test is done when the illegible “E” test is positive, to obtain objective corroboration. The examiner focuses on the optic disk and then gently moves the head as described above. If the disk moves with the head, this confirms that the VOR gain is abnormal. This test is less sensitive than the illegible “E” test.
c. HIT. The examiner stands directly in front of the patient, holds the patient’s head firmly on both sides, and instructs the patient to look at the examiner’s nose. The examiner then abruptly rotates the patient’s head laterally a small distance (approximately 10 degrees) but very rapidly; this brief but rapid rotation (the “impulse”) should be unpredictable to the patient in its direction (right or left) and timing; several impulses toward each side should be tested. In a person with an intact vestibular system, the VOR will keep the eyes on target. In a patient with a recent unilateral vestibular deficit, the eyes will “move with” the head (because of an impaired VOR), and this will be followed by a corrective “overt saccade” to bring the eyes back to the target.
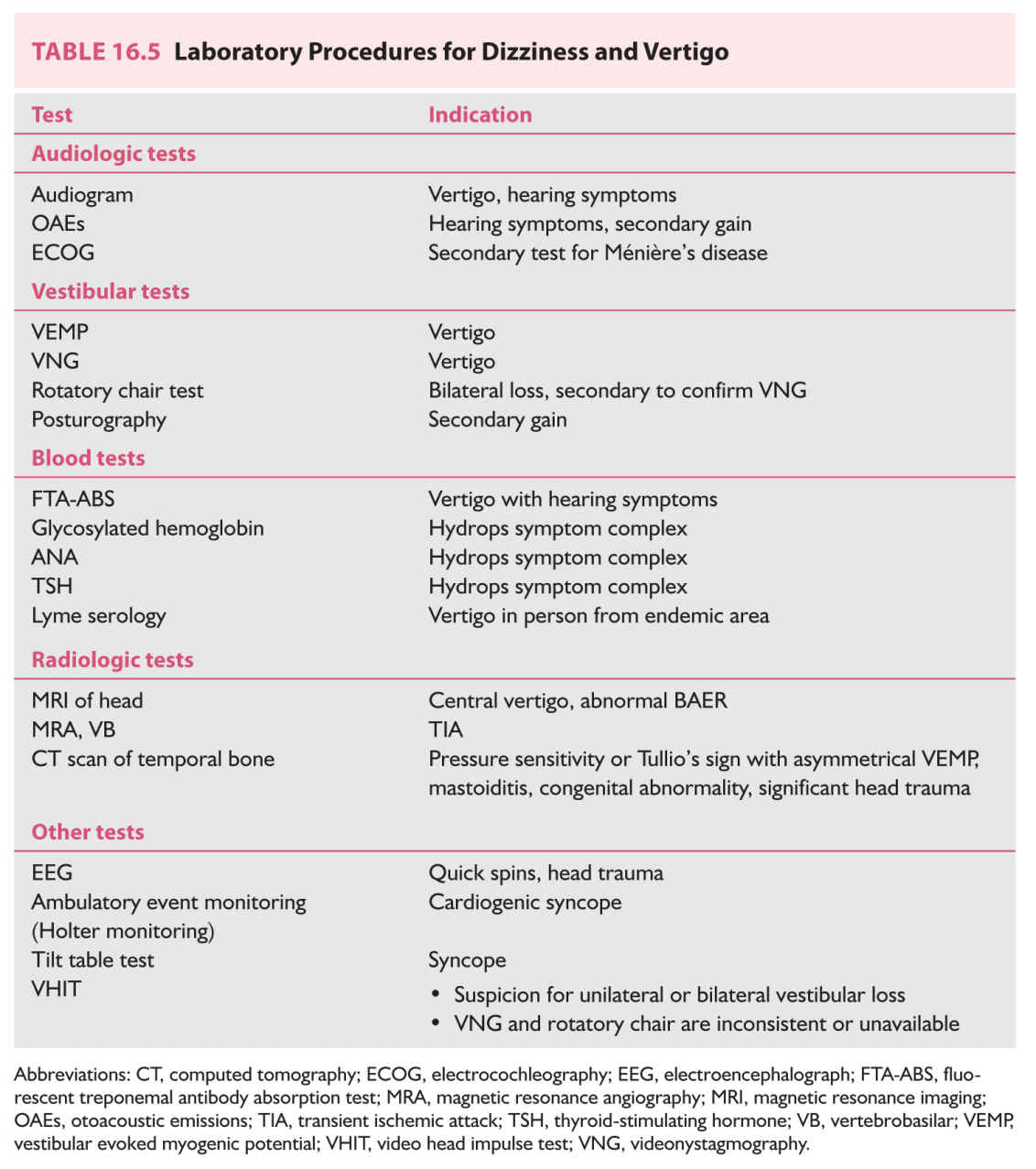
C. Laboratory studies. Table 16.5 enumerates laboratory procedures commonly used for evaluation of patients with vertigo and dizziness, with indications. For efficiency and cost containment, procedures should be selected according to specific symptom complexes and be done sequentially. Algorithms are discussed in sections Differential Diagnosis and Diagnostic Approach.
1. Audiologic testing is indicated when there are hearing complaints. If the diagnosis is uncertain audiometry is recommended even for patients who have no hearing abnormalities.
a. Audiogram. The audiogram measures hearing. Abnormalities suggest otologic vertigo.
b. Otoacoustic emissions (OAEs) measure sounds generated by the ear itself. This is a quick and simple automated procedure. OAEs are useful in detecting malingering, central hearing deficits, and persons with auditory neuropathy. In these situations, OAEs may be preserved even when subjective hearing is poor. When there is a potential for malingering, audiologists have at their disposal a large assortment of objective hearing tests that can generally detect psychogenic hearing loss. OAEs are usually not helpful in persons older than 60 years old, as OAEs are reduced with age.
c. Electrocochleography (ECOG) is an evoked potential in which the recording electrode is positioned on the ear drum. It requires that a patient have no worse than mild to moderate high-frequency hearing loss in the ear being assessed. An abnormal ECOG is suggestive of Ménière’s disease. ECOGs are difficult to perform and should not be relied upon for diagnosis by themselves.
2. Vestibular testing is not needed for every dizzy patient. The primary study—videonystagmography (VNG) or electronystagmography (ENG) test—is helpful when there is no clear diagnosis after history and examination.
a. VNG/ENG is a battery of procedures that can identify vestibular asymmetry (such as that caused by vestibular neuritis) and document spontaneous or positional nystagmus (such as that caused by BPPV). It is a long and difficult test, with little standardization, and an abnormal result that does not fit the clinical picture should be confirmed by rotatory chair testing, ideally in combination with VEMP testing.
b. VEMP testing is sensitive to SSCD syndrome, bilateral vestibular loss, and acoustic neuroma. VEMPs are generally normal in vestibular neuritis and Ménière’s disease.
c. Rotatory chair testing measures vestibular function of both inner ears together. It is highly sensitive and specific for bilateral loss of vestibular function. In unilateral loss, it is sensitive but nonspecific. Also, it does not identify the side of the lesion.
d. Video head impulse test (VHIT). This new vestibular test can quickly diagnose both bilateral vestibular loss and complete unilateral vestibular loss. It can also quantify vestibular compensation and has utility in following progress of persons undergoing treatment.
e. Posturography is an instrumented Romberg test. It is very useful in documenting inconsistency (that may be suggestive of malingering) and may also have utility in following the progress of persons undergoing treatment.
3. Blood tests are triggered by specific symptom complexes (see section Differential Diagnosis), and there is no “routine” set obtained for every dizzy patient. In particular, chemistry panels, CBCs, glucose tolerance tests, vitamin B12 levels, and allergy tests need not be routinely ordered.
4. Radiologic investigations. Skull films, cervical spine films, CT scans of the head, and CT scans of the sinuses are not recommended routinely in the evaluation of vertigo.
a. MRI scan of the brain evaluates the structural integrity of the brainstem, cerebellum, periventricular white matter, and eighth-nerve complexes. Coronal high-resolution MRI can also suggest evaluation is needed for SSCD. MRI is not routinely needed to evaluate vertigo without other accompanying neurologic findings (Chapter 32).
b. CT scan of the temporal bone provides higher resolution of ear structures than MRI and also is better for evaluating lesions involving bone (see Chapter 32). Temporal bone CT is required to diagnose SSCD. The “high-resolution direct coronal” variant of this scan is best suited for this diagnosis. The temporal bone CT scan involves considerable radiation and for this reason VEMP testing is recommended as an initial screening test for SSCD.
5. Other tests.
a. Electroencephalography (EEG) is used to diagnose seizures. Yield is very low in dizzy patients (Chapter 33).
b. Ambulatory event monitoring, or Holter monitoring, is used to detect arrhythmia or sinus arrest. Yield is high in persons with episodic orthostatic symptoms, lacking orthostatic hypotension.
c. Tilt table testing is sometimes advocated for the diagnosis of neurocardiogenic syncope. When abnormal, treatment should focus on maintenance of blood pressure.
DIFFERENTIAL DIAGNOSIS
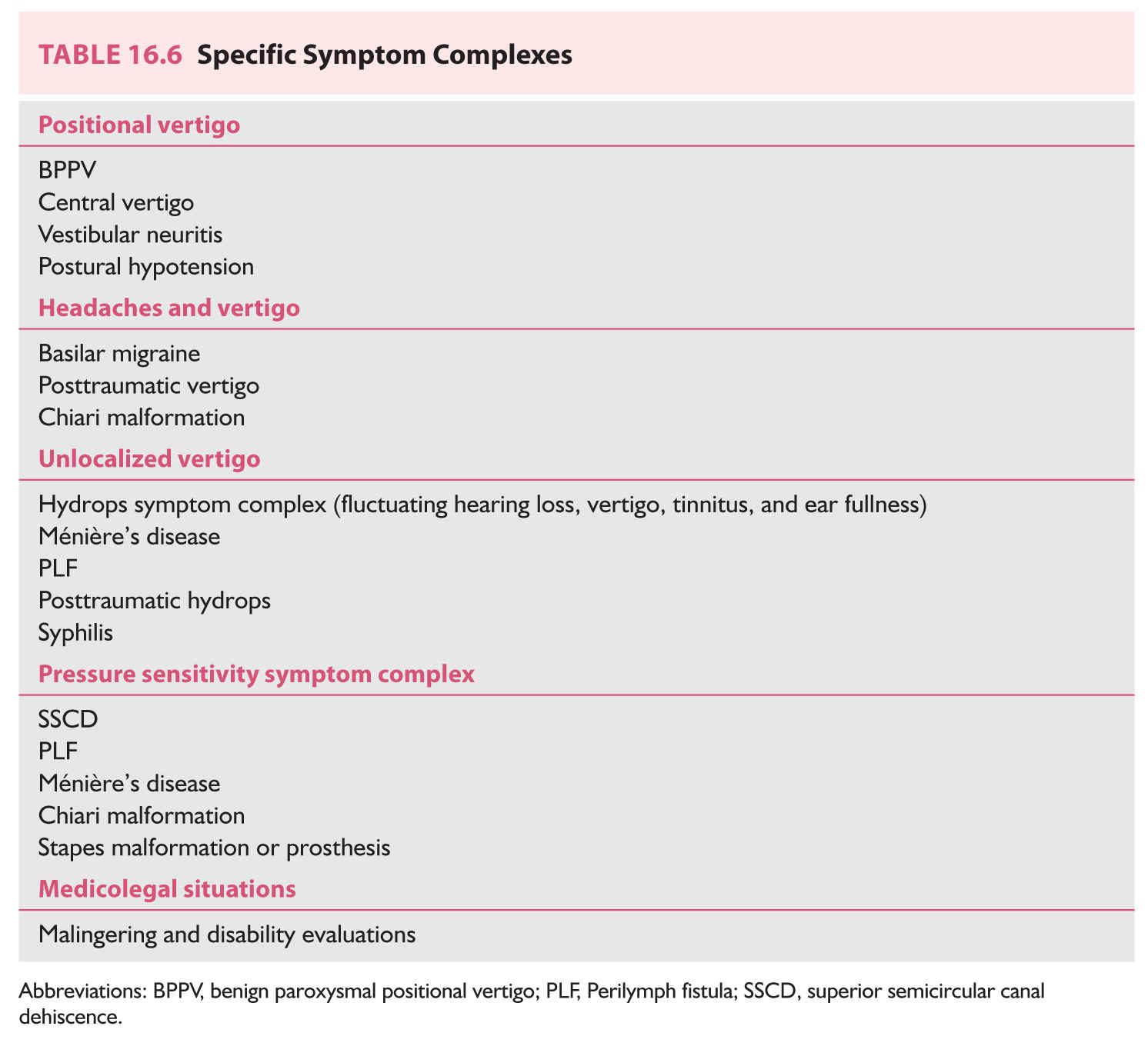
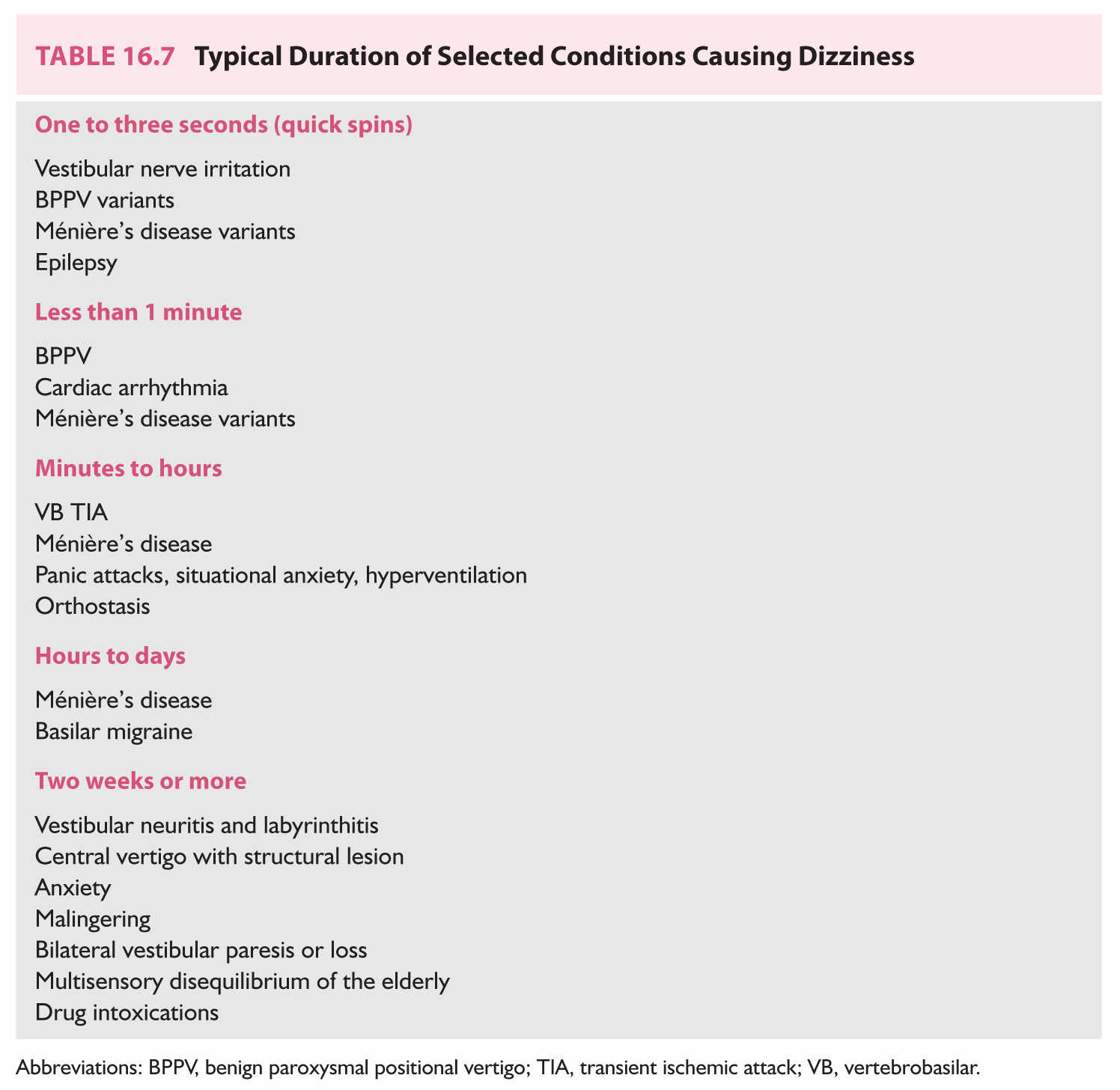
We will now discuss symptom complexes, their differential diagnosis, and algorithms used to narrow the differential. Table 16.6 enumerates five specific symptom complexes. When a patient does not fit into a complex in Table 16.6, one may fall back to grouping patients by duration of symptoms only, as in Table 16.7.
A. Approach based on specific symptom complexes.
1. Positional syndromes. Patients complain of a brief burst of rotatory vertigo when getting into or out of bed, or on rolling over from one side to the other. This symptom strongly suggests the diagnosis of BPPV.
a. BPPV. If a typical nystagmus is observed on Dix–Hallpike positional testing, no other diagnoses need to be considered. Because roughly 95% of all positional nystagmus is caused by BPPV, even in cases in which an atypical positional nystagmus is observed, it is usually most efficient to try one of the currently available treatments before considering other diagnoses. Brain MRI is indicated when an atypical BPPV is refractory to treatment.
b. Central disorders. Strong positional nystagmus may also accompany brainstem and cerebellar disorders (e.g., medulloblastoma and the Chiari malformation). Brain MRI is indicated when positional nystagmus is combined with an abnormal neurologic examination or when an atypical BPPV is refractory to treatment.
c. Vestibular neuritis. A weak horizontal positional nystagmus may be found in peripheral vestibulopathies. VNG, VHIT, and audiogram are indicated.
d. Postural hypotension also presents with dizziness on getting out of bed, but never occurs in bed. It is diagnosed by a symptomatic decrease in blood pressure between the supine and standing positions. Drops of 20 mm Hg in systolic blood pressure are significant.
2. Headaches and vertigo.
a. Migraine. A large group of patients includes women having headaches in their 30s or 50s as these are high-prevalence times for migraine. Food triggers, motion sickness, and positive family history are frequent associations. There is a weak association between BPPV and migraine, and the diagnosis of migraine-associated vertigo should prompt consideration of BPPV. Symptomatic improvement in response to empirical trials of migraine prophylaxis medication supports the diagnosis.
b. Posttraumatic vertigo. Audiometry, VNG, and CT scan of the head are indicated.
c. Chiari malformation. The headache is occipital, and there is downbeat nystagmus and ataxia. Diagnosis is from sagittal T1-MRI of the brain.
d. Unlocalized vertigo. Audiometry and VNG are indicated for the vertigo component. The headache component (tension, migraine, sinus, and so on) is considered separately.
3. Hydrops. Patients complain of spells of vertigo, roaring tinnitus, and transient hearing loss, preceded by aural fullness. Audiometry should be obtained in all patients, as well as RPR (rapid plasma reagent), fluorescent treponemal antibody absorption test (FTA-ABS), sedimentation rate, and thyroid-stimulating hormone (TSH) blood tests.
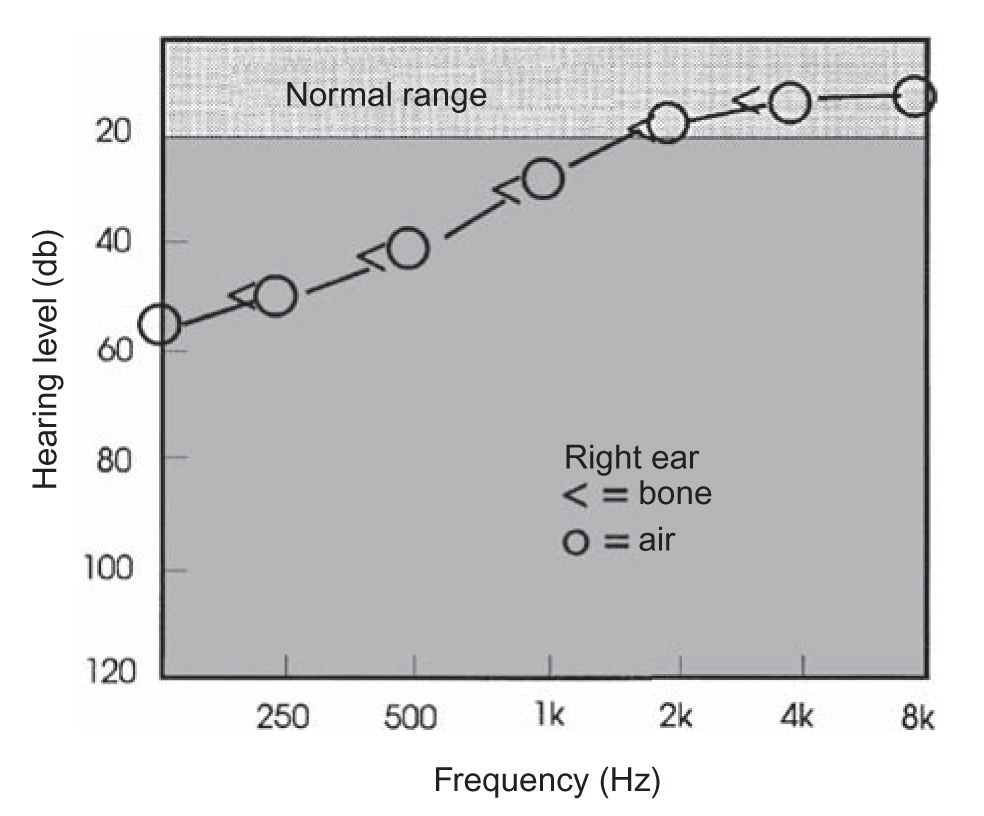
FIGURE 16.2 Low-tone hearing loss. A unilateral low-frequency sensorineural pattern hearing loss is often observed in early Ménière’s disease.
a. Ménière’s disease. Usual duration of vertigo is 2 hours, but it can vary from seconds to weeks. Audiometry is crucial to document the fluctuating low-tone sensorineural hearing loss (Fig. 16.2). The diagnosis of Ménière’s disease is highly probable when a typical history is obtained and when a fluctuating hearing loss (often in the low frequencies) is documented. ECOG testing may be performed in difficult cases, in an attempt to “rule in” the diagnosis. About 10% of all cases of bilateral Ménière’s disease are autoimmune. Thyroid disease and/or migraine are very frequent in patients with Ménière’s disease. Migraine is a comorbidity in about 50% of patients with Ménière’s disease.
b. Perilymph fistula (PLF). Occasionally, fistula presents with hydrops rather than the pressure sensitivity symptom complex (see Section A.4 under Differential Diagnosis). The only clue may be a history of barotrauma.
c. Posttraumatic hydrops is a variant of the Ménière’s disease symptom complex that appears after a significant blow to the ear, with presumed bleeding into the inner ear. It is very rare.
d. Syphilis. Hearing loss is bilateral. Diagnosis is by rapid plasma reagent followed by FTA-ABS.
4. Pressure sensitivity. Patients complain of dizziness or ataxia evoked by nose blowing, high-speed elevators, cleaning of the ear with a cotton swab, straining as at stool, after the landing of an airplane, or after diving. In addition to pressure sensitivity, patients report vertigo induced by loud noises (Tullio’s phenomenon) and by exercise. Patients are often extremely motion-intolerant and visually sensitive. Audiometry and the VEMP test are indicated.
a. SSCD syndrome is the main source of pressure sensitivity. Vertigo and nystagmus can be provoked by loud noise or pressure. This syndrome is caused by dehiscence of bone overlying the superior semicircular canal. VEMP testing is nearly always abnormal because of both asymmetry and a low threshold on the dehiscent side. Diagnosis is confirmed by a high-resolution CT scan of the temporal bone.
b. PLF. Most patients have a history of barotrauma in they were unable to “clear” their ear during scuba diving or airplane travel. Audiometry and ECOG are indicated. A trial of a “ventilation” tube in the suspect ear is often helpful.
c. Ménière’s disease. Mild pressure sensitivity occurs in about one-third of patients with Ménière’s disease. See the hydrops symptom complex description (see Section A.3 under Differential Diagnosis) for a differential diagnosis.
d. Chiari malformation and platybasia. Vertigo is correlated with straining but not with pressure in the external ear canal. The downbeat nystagmus and abnormal MRI found in the Chiari malformation also separate it from the other entities.
e. Stapes malformation. Remarkable pressure sensitivity with torsional movement of the eye occurs in patients in whom stapes prostheses (for otosclerosis) of excessive length have been inserted, or migrated into the inner ear. A high-resolution CT scan of the temporal bone is indicated in this situation.
5. Medicolegal situations. The possibility of malingering often comes up in disability evaluations, worker’s compensation cases, and legal situations where patients may potentially be compensated for being vertiginous. These patients usually present no objective evidence on physical examination or testing. Often they resist examination, by closing or crossing their eyes at inappropriate times or by refusing to perform key positional maneuvers. Their complaints often cannot be resolved into one of the symptom complexes discussed above. Objective testing (audiometry, OAE, VNG, VEMP, VHIT and/or rotatory chair, and an MRI scan of the head) is nearly always appropriate. In addition, posturography may be helpful. This test can help detect the malingering patient by assessing balance in a series of test conditions of graded difficulty, but presenting those conditions in a random order. The malingerer who is trying to fail the posturography test will frequently perform equally poorly on the easy and difficult subtests, producing a pattern that is neither normal nor typical of vestibular deficits.
B. Approach based on timing only. These categories (Table 16.7) are less useful for diagnosis than those based on symptom complexes, but can be used when patients do not fall into any category.
1. Quick spins are brief spells (1 to 3 seconds) of true vertigo, unaccompanied by secondary symptoms. EEG should be obtained. A trial of oxcarbazepine may be helpful.
a. Vestibular nerve irritation due to the microvascular compression syndrome or a residual from vestibular neuritis. Spells are very brief (often just a fraction of a second) but can be extremely frequent (such as 50 per day). Hyperventilation may induce nystagmus seen with video Frenzel’s goggles. Magnetic resonance angiography (MRA) occasionally documents compression of the brainstem by the vertebrobasilar arterial system, but there are many false positives. If the EEG is normal, a good response to oxcarbazepine confirms the diagnosis.
b. Ménière’s disease variants. Patients complain of “shocks” or “earthquake” sensations. Frequency of spells is daily at most. Hearing is often affected. For diagnosis, see hydrops symptom complex (see Section A.3 under Differential Diagnosis).
c. BPPV variants. Spells are of no more than daily frequency. Presumably, otoconial debris are caught on a canal wall and suddenly slip down. Diagnosis is by the Dix–Hallpike maneuver. It may take several visits to get a positive result.
d. Epilepsy. Spells can be frequent, and there is often a history of head injury. Cognitive impairment is frequent during the dizzy spell.
2. Less than 1 minute. These are mainly postural syndromes.
a. Classic BPPV. If there is a history of positional vertigo, this diagnosis is easy. However, poor “historians” may omit to mention that they have adopted sleeping strategies (e.g., two pillows) by which bed spins are avoided. BPPV can also be triggered by unusual head positions such as looking up at the “top shelf.” Diagnosis is by the Dix–Hallpike maneuver.
b. Cardiac arrhythmia. The clue is usually that vertigo spells occur mainly while standing, and that lightheadedness is a more prominent symptom than spinning. Ambulatory event monitoring is the best method of documenting this problem. Holter monitoring may be used in contexts where event monitoring is not available.
c. Ménière’s disease variants. See Section B.1.c under Differential Diagnosis.
3. Minutes to hours.
a. TIA. Spells of pure vertigo lasting 2 to 30 minutes, of abrupt onset and offset, in a patient with significant vascular risk factors are diagnosed as VB TIA until proven otherwise. Suspicion is reduced if there is a positional trigger. MR angiography (MRA) and CT angiography (CTA) of the VB circulation are the most useful tests.
b. Ménière’s disease. The typical Ménière’s attack lasts 2 hours. If there are hearing symptoms, see the hydrops symptom complex (see Section A.3 under Differential Diagnosis). If not, be cautious about proposing this diagnosis. Sometimes the term “vestibular Ménière’s disease” is used to denote episodic vertigo having the typical timing of classic Ménière’s disease but without any ear symptomatology. It is presently unclear whether this entity exists, and there is no method of confirming this diagnosis.
c. Panic attacks, situational anxiety, and hyperventilation may produce symptoms of this duration (minutes to hours). These patients ordinarily are not symptomatic during examination. A detailed history is the most useful diagnostic test. If hyperventilation reproduces symptoms in patients without other findings, the diagnosis is hyperventilation syndrome. If hyperventilation also induces nystagmus, MRI is indicated.
d. Cardiac arrhythmia and orthostasis. See Etiology C1, C2 and Evaluation, C5c.
4. Hours to days.
a. Ménière’s disease.
b. Migraine. Migraine is so common in the general population that even unusual variants, such as manifestation solely as a vertiginous aura or intractable motion sensitivity with nausea, are relatively common. Diagnosis is suggested by age, female gender, positive family history, attacks provoked by usual migraine triggers, and sensitivity to multiple sensory triggers (e.g., light, sound, motion).
5. Two weeks or more.
a. Vestibular neuritis. Diagnosis is made by combining a long duration of symptoms, typically more than a week, with spontaneous nystagmus or an abnormal VNG or VHIT test. The VNG should document nystagmus or a significant vestibular paresis (a conservative criterion is a paresis of 40% or more). The VHIT test should be positive for a unilateral weakness. The VEMP test should be normal. After 2 months of vertigo, central vertigo becomes more likely and an MRI is indicated. For labyrinthitis, the diagnosis is made by combining the vestibular neuritis pattern with hearing symptoms. Audiometry, erythrocyte sedimentation rate, and fasting glucose are indicated in addition to the vestibular neuritis battery.
b. Central vertigo with a fixed structural CNS lesion. This diagnosis should be considered when there are neurologic symptoms or signs accompanying vertigo. Central vertigo may be permanent. For example, the combination of a peripheral vestibular loss with a cerebellar lesion may occur after acoustic neuroma surgery. Nevertheless, acoustic neuromas are extremely uncommon sources of peripheral or central vertigo owing to their rarity compared with disorders such as BPPV. MRI is the most effective method of diagnosis of central vertigo. There are no examination maneuvers that can always separate peripheral vertigo (such as due to vestibular neuritis) from a central vertigo that lacks any “central signs.”
c. Anxiety. With this duration of symptoms (2 weeks or more), patients may be complaining of vertigo in your office. If a patient is presently complaining of vertigo, but no spontaneous nystagmus is evident under Frenzel’s goggles, if they are not taking vestibular suppressants, one may reasonably conclude that the vertigo is functional in origin. Patients with anxiety typically report that nearly every trigger factor in Table 16.4 exacerbate their symptoms. Interestingly, whereas most patients with inner ear problems report that stress makes their symptoms worse, patients with anxiety frequently claim that everything except stress triggers vertigo. A positive response to a trial of a benzodiazepine supports this diagnosis but does not establish it because many organic vestibular disorders also respond well to these medications.
d. Malingering. Malingerers persist in reporting symptoms as long as necessary to accomplish their purpose of obtaining favorable court settlements or disability rulings. Posturography and neuropsychological testing is usually very abnormal. Objective tests of vestibular function such as VHIT, VEMP, and VNG are nearly always normal. Tests that are more vulnerable to lack of cooperation such as rotatory chair tests are variable.
e. Bilateral vestibular paresis or loss. These patients fail the dynamic illegible “E” test and the eyes-closed tandem Romberg’s test. Their ataxia is worse in the dark. On audiometry, hearing is usually normal. Rotatory chair testing or VHIT testing is the best way to confirm this diagnostic impression.
f. Multisensory disequilibrium of the elderly is essentially an unlocalized ataxia in an elderly patient. If the diagnosis is accurate, this is usually a permanent condition.
g. Drug intoxications. Diagnosis depends on a positive response to withdrawal of medications.
DIAGNOSTIC APPROACH
A. Perform history and examination as outlined in sections Clinical Manifestations and Evaluation.
B. Approximately 20% to 40% of patients are diagnosed immediately on examination.
1. BPPV patients on Dix–Hallpike maneuver (15% to 20% of vertigo population).
2. Orthostatic hypotension and fixed cardiac arrhythmia such as atrial fibrillation (2% to 5%).
3. Bilateral vestibular paresis or loss on dynamic illegible “E” test (5%).
4. SSCD with positive Valsalva’s test (0% to 2%).
5. Acute vestibular neuritis via spontaneous nystagmus and positive HIT test (2% to 5%).
C. For the remaining patients, proceed as follows:
1. If patient fits into a symptom complex category, follow procedures presented in Section A under Differential Diagnosis.
2. If patient does not fit into a symptom complex, follow procedures outlined in Section B under Differential Diagnosis.
a. If symptoms are intermittent, follow procedures in Sections B.1 to B.3 under Differential Diagnosis.
b. Otherwise, if symptoms are constant, proceed as follows:
(1) If duration has been <2 weeks, treat symptomatically or simply reassure and have patient return if symptoms persist beyond 2 weeks.
(2) If duration has been >2 weeks, follow the procedures outlined in Section B under Differential Diagnosis.
A. Otology.
1. Cerumen disimpaction and ear microscope examination. Ear wax can be safely removed with the examining microscope, a standard piece of otologic equipment.
2. Progressive or acute hearing loss has potential medicolegal ramifications, and otologic consultation is often helpful.
3. A perforated tympanic membrane or mass in the canal or behind the tympanic membrane may require otologic referral for closure of the perforation or surgical management of the tumor.
4. Mastoiditis or chronic otitis media. These patients are commonly managed with a mixture of surgery, cleaning, antibiotics, and antiseptics that requires otologic supervision.
5. Surgical management of acoustic neuroma, Ménière’s disease, fistula, SSCD, and cholesteatoma. Surgical treatment for Ménière’s disease has greatly improved in recent years because of greater use of low-dose gentamicin protocols.
B. Internal medicine.
1. Cardiac or blood pressure problems, especially arrhythmia.
2. Management of diabetes or thyroid dysfunction.
C. Psychiatry.
1. Patients with disabling anxiety or panic disorders.
D. Neuropsychology.
2. Patients who may be malingering.
E. Vestibular rehabilitation (physical therapy).
1. Treatment for BPPV, bilateral loss, and refractory vestibular neuritis.
2. Video Frenzel’s goggle exam (if examiner does not have this critical piece of equipment).
Key Points
• Dizziness can be broadly categorized as otologic (common causes: BPPV and vestibular neuritis), central (common causes: migraine-associated vertigo), medical (common causes include postural hypotension, adverse effects of medication), and unlocalized (including posttraumatic and psychogenic).
• The most common causes of dizziness in adults include BPPV, migraine-associated vertigo, and vestibular neuritis. The most common cause of dizziness in children is migraine-associated vertigo.
• Aside from the history, general, otologic, and neurologic examinations, the differential diagnosis often relies crucially on clinical examination of eye movements, sometimes supplemented by otologic testing (such as an audiogram) and vestibular testing (such as VEMPs, VNG, and rotatory chair testing).
• Bedside examination maneuvers with high yield include observation for spontaneous unidirectional nystagmus (suggesting vestibular neuritis) and the Dix–Hallpike maneuver (to diagnose posterior canal BPPV).
• Imaging may be helpful in cases where screening otologic and vestibular workups are unrevealing, where specific neuroanatomical abnormalities are suspected (such as Chiari malformation), where medicolegal factors are involved, or when malingering is suspected. Suspicion of acoustic neuroma or congenital inner ear abnormalities warrants MRI of the brain and internal auditory canals without and with contrast. In cases where SSCD is suspected, or where there has been significant head trauma, a temporal bone CT without contrast is appropriate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree