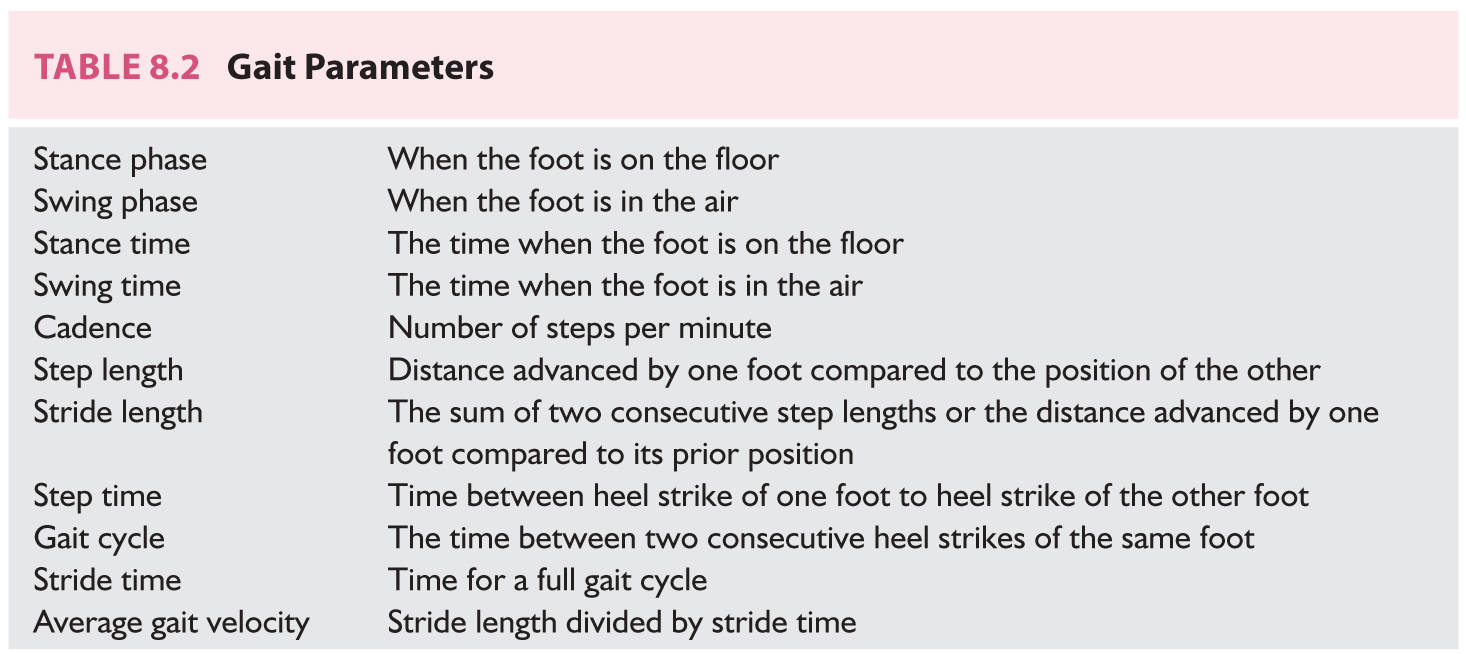
FIGURE 8.1 Summary of important areas involved in human locomotion. Shaded areas are hypothesized to be important in the development of HLGDs. CPGs, central patterns generators; HLGD, high-level gait disorder; MLR, mesencephalic reticular formation; PMRF, pontomedullary reticular formation. (From Nutt JG. Higher-level gait disorders: an open frontier. Mov Disord. 2013;28(11):1560–1565.)
3. Cortical locomotor areas. The premotor (PM) and the supplementary motor area (SMA) are very important for gait initiation. PM cortex may be responsible for sensory-guided gait initiation, whereas the SMA may be also important for postural control. The temporoparietal cortex may integrate inputs from visual, vestibular, and proprioceptive pathways that would help to generate, in real-time, motor programs by the PM and SMA to adapt to a changing walking environment or situation.
ETIOLOGY
A. Gait disturbances. A gait disturbance could be understood as a gait pattern that deviates from the accepted “normal” gait; in that sense defective synchrony, fluency, smoothness and symmetry while walking, among other features, would represent an abnormal gait. A gait disturbance may be caused by disruption at any level of the neuraxis, and following the proposal by Nutt, Marsden, and Thompson gait disorders can be classified in a hierarchical anatomically based system. This classification divides gait disorders into low-level, middle-level, and high-level gait disorders (HLGDs) (Table 8.1). It should be taken into account that multiple factors are often at play to cause gait disturbance, particularly in the elderly.
B. Causes of recurrent falls. Errors in judgment and environmental hazards are responsible for one-third to one-half of the falls. About one-third of people older than 65 years may fall at least once a year, with one-fourth of them suffering a serious injury and about 5% of them having a fracture. Most of the patients with recurrent falls have neurologic disease, and the incidence of falls in hospitals and nursing facilities is almost three times the rates for community-dwelling older adults over 65 years of age. Common cause of recurrent falls include peripheral neuropathy, residual of stroke, diffuse cerebral ischemic disease, parkinsonian syndromes, dementing illnesses, effects of medication, orthostatism, vestibulopathy, and poor vision, among others.
CLINICAL MANIFESTATIONS
A. Features of normal walking. The gait cycle is defined as the time between successive heel–floor contacts with the same foot, and it consists of two steps that would be one stride (Fig. 8.2). The gait cycle can be divided into two phases: stance phase and swing phase. About 60% of the gait cycle is spent in the stance phase and 10% of that time is bipedal support. The stance phase is when the foot is on the ground; the swing phase is the time when the foot is in the air. The stance phase can be divided into initial double-limb support, single-limb stance, and second double-limb support. The swing phase is divided into initial swing, mid swing, and terminal swing. Gait parameters such as step length, cadence, and step height are shown in Table 8.2.
1. Stride length (length of two successive steps) and cadence (steps per minute) determine the velocity of walking. Gait velocity and step length are lower among women while cadence is higher in women. The most commonly affected gait parameters in older individuals are the reduction of walking speed and step length and increase in bipedal support. The magnitudes of arm swing, toe–floor clearance, and hip and knee rotations are proportional to stride length and velocity. Reduced gait velocity is a nonspecific sign of an underlying medical condition and is associated with reduced long-term survival.
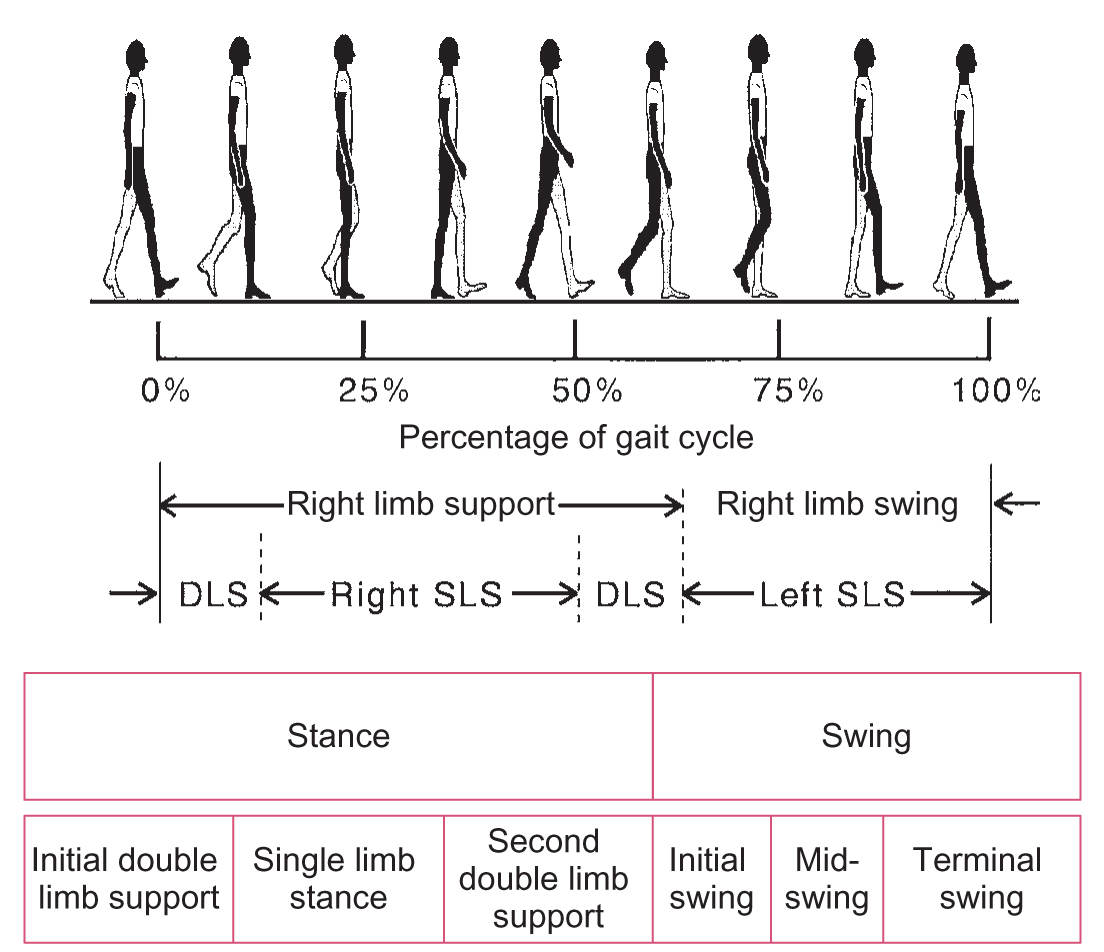
FIGURE 8.2 Phases of the normal gait cycle, expressed as percentage of total stride. DLS, double-limb support; SLS, single-limb support.
2. The total-body center of mass oscillates vertically and horizontally during ambulation. The maximal vertical oscillation happens during the single-limb support, while the most lateral horizontal excursions of the center of gravity occur at the times of mid-single-limb support. These vertical and horizontal excursions of the center of mass are optimized to consume the least amount of energy. Therefore, most gait disorders are associated with increased energy expenditure during ambulation.
B. Normal development of walking. Most children walk independently by 15 months, and failure to walk by 18 months should be investigated as it could be indicative of an underlying central nervous system (CNS) pathology, neuromuscular disease, visual impairment, or vestibular disease. Maturation of gait and balance probably continues throughout childhood, but most of this maturation is accomplished by 3 or 4 years of age. By 5 years, the child should be able to walk, run, hop, skip, and jump.
C. Abnormal patterns of walking. Gait abnormalities can be classified in a hierarchical way as emerging from disturbances at different levels of the neuraxis and neuromuscular system. With this in mind, we may divide gait disorders as lower-, middle-, and higher-level gait disorders on the basis of the levels of motor control of locomotion. It should also be taken into account that the characteristics of an abnormal gait are usually a combination of the underlying pathologic process, compensatory mechanisms, and potential development of secondary musculoskeletal abnormalities.
1. Lower-level gait disorders
The clinical characteristics of lowest- and middle-level gait disorders are predictable and consistent with the observed neurologic and neuromuscular deficits. The associated compensatory mechanisms are appropriate although limited by the underlying pathologic process and do not have a maladaptive counterproductive quality. Lower-level gait disorders are caused by disease of the muscles, peripheral nerves, skeletal pathology, peripheral vestibular system, and anterior visual pathway.
a. Steppage gait. Steppage gait is seen in the context of weakness of foot dorsiflexion and could be uni- or bilateral. For the toes to clear the ground during the swing phase of gait and to avoid tripping, it will require an exaggerated knee and hip flexion on the affected leg; the step is high and short, and the foot commonly slaps the floor at the end of swing phase. Peroneal nerve injury, radicular compromise, and a demyelinating neuropathy are common causes of this gait pattern.
b. Waddling and Trendelenburg gaits. The waddling gait pattern can be seen with bilateral hip girdle muscle weakness and in patients with bilateral orthopedic hip problems. Gait is wide based, short stepped, and associated to increased alternating lateral body sway and excessive hip drop. Increased arm abduction may also occur as well as exaggerated lumbar lordosis if there is superimposed weakness of the paraspinal muscles. Trendelenburg gait is characteristic of unilateral hip abductor weakness, and it is associated with ipsilateral lurching of the torso and hip drop on the contralateral side while standing on the leg of the affected side.
c. Sensory ataxia. The ataxic gait is characterized by wide base with variability in step length, and there is considerable stride-to-stride variability. This is associated with unsteadiness, and it is not specific for a particular location in the neuraxis: it can be seen in the context of proprioceptive deficits (i.e., sensory ataxia), cerebellar dysfunction (i.e., cerebellar ataxia), and pontine and thalamic pathology. The problem is more obvious when the eyes are closed (Romberg test). Abnormal postural sway can be present during stance with eyes open and closed.
d. Visual disequilibrium. Sensation of being off balance in the context of acute visual distortion such as when putting new prescription glasses on. This is typically associated with increased base of support, cautiousness, and tentative steps.
e. Vestibular disequilibrium. Acute vestibular dysfunction may cause vertigo, with potential nystagmus, instability, and a tendency to veer or fall toward the affected side. When the problem is chronic, the symptoms are usually less dramatic, but the gait tends to be still cautious and mildly wide based, and the patient has difficulty with Romberg maneuver and tandem walking. Patients are able to stand and walk without assistance despite their disequilibrium.
f. Sensory disequilibrium. It refers to feelings of unsteadiness when there is a conflict among inputs from visual, proprioceptive, and vestibular pathways. Acutely, this can be associated with falls but it may become chronic specially if there is significant loss of at least two of these sensory modalities or there is no adjustment of the CNS to the conflictive information from the different sensory modalities. In this setting, the gait is slow and cautious, with increased bipedal support.
2. Middle-level gait disorders
Middle-level gait disorders are caused by ascending or descending sensorimotor tract lesions, cerebellar dysfunction, bradykinesia, and hyperkinetic movement disorders.
a. Spastic gait and scissoring. Lesion of the corticospinal tracts causes spastic gaits that can be hemiparetic or paraparetic depending if the lesion is uni- or bilateral. In spastic gaits there is often a component of associated weakness. Spastic hemiparetic gaits are characterized by lower limb hyperextension with difficulty to flex hips and knees and excessive plantar flexion and inversion of the foot. Arm swing is reduced with flexor or dangling arm posture. The base of support is narrow and because of the hyperextension of the leg, to clear the ground while walking, will require a semicircular movement at the hip during the swing phase (circumduction; ![]() Video 8.1). Patients with little spasticity and appropriate proximal strength may clear the floor during the swing phase with increased hip flexion. Spastic paraparesis is more commonly seen in patients with spinal cord injuries and shares some features with hemiparetic gaits. In a sense, it could be imagined as walking on water up to the waist level. In addition, in many cases, arm swing and upper limb posture are relatively normal depending on the level of the pyramidal tract compromise. Spastic diplegic gait could be understood as a bilateral hemiparetic gait with some particular features; the knees and hips are significantly flexed and hips are also adducted during the gait cycle, causing the legs to cross each other in a pattern termed “scissoring.” Compared to bilateral hemiparesis in adults, in spastic diplegia the upper extremities and bulbar musculature are usually much less affected than the lower extremities.
Video 8.1). Patients with little spasticity and appropriate proximal strength may clear the floor during the swing phase with increased hip flexion. Spastic paraparesis is more commonly seen in patients with spinal cord injuries and shares some features with hemiparetic gaits. In a sense, it could be imagined as walking on water up to the waist level. In addition, in many cases, arm swing and upper limb posture are relatively normal depending on the level of the pyramidal tract compromise. Spastic diplegic gait could be understood as a bilateral hemiparetic gait with some particular features; the knees and hips are significantly flexed and hips are also adducted during the gait cycle, causing the legs to cross each other in a pattern termed “scissoring.” Compared to bilateral hemiparesis in adults, in spastic diplegia the upper extremities and bulbar musculature are usually much less affected than the lower extremities.
b. Cerebellar ataxia occurs typically in patients with bilateral damage to the cerebellum. Gait is wide based with significant stride-to-stride variability. Upper and lower limb movements are uncoordinated and abnormal postural sway during quiet stance is present. Different patterns of gait abnormality can be seen depending on what regions of the cerebellum are involved (i.e., lateral, intermediate, and medial functional zones). The medial zone (vermis) regulates extensor tone and dynamic balance control, and modulates the rhythmicity of flexor and extensor muscles. Therefore, a rostral vermian and paravermian lesion produces a predominantly trunkal and lower limb ataxia. This pattern of cerebellar degeneration is typically seen in chronic alcoholism and chronic thiamine deficiency. Caudal midline vestibulocerebellar damage produces disequilibrium and eye movement abnormalities. Patients tend to fall toward the side of the cerebellar or central vestibular lesion. Damage to the vestibular nuclei can produce a sensation that the environment is tilted and that the body is being pulled toward the side of the lesion. The intermediate cerebellar zone is important while performing precise goal-directed limb movements. The lateral cerebellum plays a significant role in adjusting to a new context or when strong visual guidance is required during ambulation but it seems to be less important in uninterrupted walking.
c. Parkinsonian gait. Short-stepped shuffling gaits are often referred to as parkinsonian gaits and are typically seen among patients with frontal lobe and basal ganglia dysfunctions. The typical gait in Parkinson’s disease is narrow-based with short and slow steps and associated with stooped-over posture, stiff trunk, and diminished asymmetric arm swing. Turns are characteristically slow, requiring multiple steps, often more than four or five steps. Festination is the tendency to walk faster and faster with diminished step length, like trying to chase the center of gravity, and it is often seen in patients with freezing of gait (FoG). In cases of cerebrovascular disease with parkinsonian-like gait the base of support tends to be wider than in classic Parkinson’s disease and arm swing has higher amplitude than the step length.
d. Choreic gait is characterized by superimposed abnormal hyperkinetic movements on top of the normal gait pattern, causing random extremity and trunk movements and postural shifts that can give the gait a bizarre, dance-like appearance (“chorea”). Choreic gait is often associated to increased risk of falling.
e. Dystonic gait is a pattern of walking in which extremity and trunkal movements and postural shifts are interrupted by tonic or phasic co-contractions of antagonistic muscles in a fairly stereotypic fashion. There is often some foot intorsion and the disorder can be task specific, making walking backward sometimes easier than walking forward. The abnormal postures and patterns observed while walking will depend on the extent of the dystonic disorder and give the gait a bizarre appearance.
3. Higher-level gait disorders
HLGDs are caused by impairment of the cortical–basal ganglia–thalamocortical pathways. Over the years multiple terms have been used to describe these walking difficulties, such as lower-body parkinsonism, gait apraxia, magnetic gait, or frontal lobe gait disorders to name a few. HLGD is not a single entity and it encompasses several potential gait patterns that are characterized by some of the following features:
a. Bizarre or inadequate postural synergy, limb and trunkal placement, and interactions with the environment (e.g., leaning backward when trying to get up from a chair).
b. Poor or absent rescue responses to postural perturbation.
c. Variable performance based on changes in the environment or emotional status.
d. Freezing or hesitation with seemingly insignificant environmental challenges (e.g., FoG while going through a doorway).
Bilateral lesions produce more significant disturbances of gait and it is often seen in parkinsonian syndromes and dementias as well as in extensive cerebrovascular disease. Unilateral lesions, on the other hand, cause a tendency to fall away from the lesion.
Some HLGD gait patterns:
1. Cautious gait. It is a gait pattern characterized by a widened base, slow speed, and diminished step length that resembles someone walking on ice. This is seen when an individual is walking in response to a real or perceived lack of balance and it is more commonly seen in elderly people. It is associated with slightly stooped posture, increased double-limb support, widened base, and reduced arm swing. This pattern can be seen in patients with cerebrovascular disease and neurodegenerative disorders but, unfortunately, this pattern of walking is nonspecific and provides with no significant clues to understand the underlying pathologic process.
2. Fear of falling. This is a maladaptive behavior in which perceived disequilibrium or previous falls have triggered an abnormal gait pattern. This is characterized by a tendency to grab or hold onto other people, walls, or furniture when trying to walk for fear of falling. This fearfulness is very often out of proportion to the actual walking abilities and it can be very limiting to the point that the patient may refuse to walk.
3. FoG. It is an episodic and brief spell of absence or significant reduction of forward progression while trying to walk to the point that feet may seem to be glued to the ground. Patients move their extremities relatively normally while seated or recumbent, but their feet appear to stick to the floor while walking. When the difficulty happens as the patient is trying to initiate gait it is called start hesitation or gait ignition failure; if it happens before getting to a target destination it is called destination hesitation. Environmental distractions and obstacles such as going through a doorway exacerbate or trigger FoG.
4. Frontal and subcortical disequilibrium. Disequilibrium due to acute unilateral frontal lobe lesions causes patients to fall away from the side of the lesion; lesion in the basal ganglia, ventrolateral thalamus, or dorsolateral midbrain causes a tendency to fall backward and laterally away from the lesion. However, when the lesions affect both frontal lobes or the subcortical structures just mentioned, the disequilibrium is more profound and sustained. This disequilibrium is characterized by poor synergy between postural and locomotor abilities and they are associated to inappropriate and very often counterproductive adjustments; patients may lean the wrong direction in such a way that stability and locomotion are compromised. This causes difficulty keeping balance while standing and potentially while sitting as well.
4. Other gait disorders
a. Antalgic gait. Stance and gait are modified to reduce pain and decrease time in the stance phase on the affected limb is the norm. This is associated commonly to limping. The actual gait pattern would change depending on the location of the pain, for example, with hip pain it will be the so-called “abductor lurch” with shifting of the upper torso toward the painful hip during the single-limb stance on the painful hip and there is no hemipelvis drop like the one seen in Trendelenburg gait. In addition to pain, skeletal deformities are also associated to changes in stance and walking.
b. Functional/psychogenic gait. Psychiatric disorders such as depression and schizophrenia may affect walking and balance; very often these patients walk slower and with diminished step length. Conversely, patients with psychogenic gait disorders tend to display bizarre and variable gait patterns that do not fit with known patterns of organic gait disorders. The most common patterns include excessive slowness, buckling of the knees, and acrobatic-like gait (i.e., astasia–abasia). Patients frequently lean, lurch, and gyrate in a manner that requires good balance and coordination. With distraction or suggestion patient’s gait may improve even if this is more challenging than the original spontaneous gait observed (e.g., asking patient to walk on heels or toes; reciting months of the years backward while walking). Sudden onset of symptoms, a paroxysmal course, inconsistent and changing gait patterns, or acrobatic-like postures or gait while retaining the ability to perform quick steady normal turns should prompt considering a functional etiology. However, the clinician should exercise caution when making this diagnosis as some brain disorders may be associated to bizarre-appearing gaits.
EVALUATION
A. History is critical in determining the cause of the gait difficulties and it should be focused on the nature of the problem, timing, modifying factors, falls, and comorbidities that may have a negative impact on the ability to walk. This is important to establish the cause and potentially suggest the most appropriate workup and therapeutic approach in patients with gait difficulties.
1. Functional disability is largely determined with a careful history. The frequency and circumstances of falls and the ability to perform various activities of daily living (dressing, bathing, climbing stairs, and getting in and out of bed and chairs) are important measures of disability.
2. Associated signs and symptoms may suggest the cause such as rest tremor in Parkinson’s disease, vertigo in vestibulopathies, urinary incontinence in patients with normal pressure hydrocephalus (NPH) or multiple system atrophy (MSA), paresthesia in patients with neuropathy or muscle wasting in patients with neuromuscular disease, for instance.
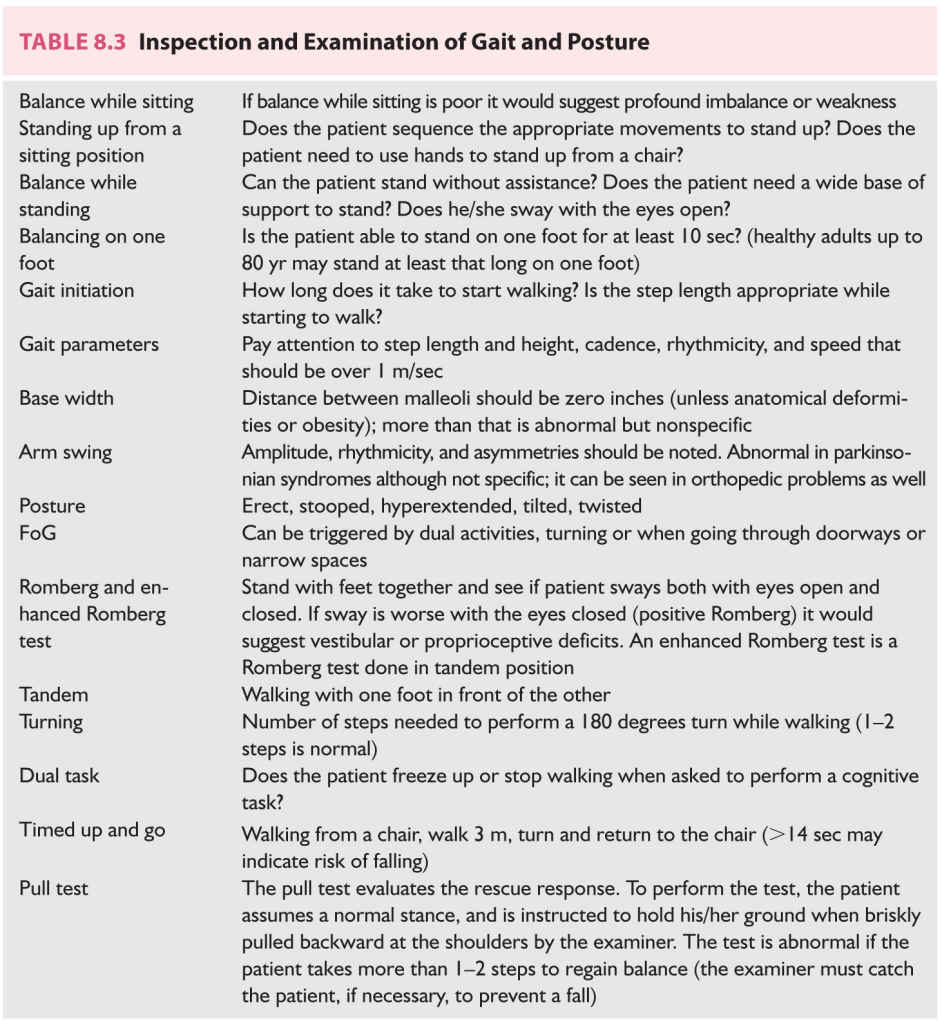
B. Physical examination is aimed at trying to anatomically localize the lesion and establish the degree of disability. This should include a full neurologic exam and a general physical exam emphasizing the observation of gait parameters, posture, range of motion tandem walking, and the use of provocative tests such as the Romberg tests (Table 8.3). A detailed vestibular examination may be indicated in some patients (Table 8.4). Attention should be paid to localizing signs such as pyramidal signs, tremor, sensory changes, or dysmetria, for instance. The general examination should also look for physical signs of musculoskeletal problems, poor eyesight, cardiovascular diseases, and the possibility of orthostatism that could impact balance and walking.
C. Laboratory studies help to confirm the cause of the suspected gait disorder or to clarify the differential diagnosis inferred from the history and physical exam. The clinical suspicion should guide the clinician when deciding what studies are appropriate to answer the question at hand.
1. Blood work including a complete blood count, chemistry panel, and thyroid, renal, and liver function studies are frequently performed. Based on the clinical findings a vitamin B12 level is appropriate in cases of neuropathy, suspected subacute combined degeneration of the spinal cord, or elderly patients with cognitive difficulties and gait problems, for instance. Multiple other blood tests may be appropriate depending on the clinical suspicion. A lumbar puncture would be indicated in patients with suspected NPH to drain between 40 and 60 mL of cerebrospinal fluid (CSF) (i.e., Fisher’s test) and establish if this would improve their ability to walk.
2. Imaging studies such as brain MRI and head computed tomography (CT) scans are performed as needed, looking at the brain for enlarged ventricles, space-occupying lesions such as subdural fluid collection, old ischemic strokes, cerebral atrophy, and diffuse white matter changes. Evaluation of the spine may uncover evidence of space-occupying lesions, spinal stenosis, or spinal deformities that may cause walking difficulties. In cases of suspected degenerative parkinsonian syndromes a dopamine transporter scan may shed some light and show diminished uptake of the radioactive ligand in the striatum.
3. Radiography of the hips, spine, and extremities is performed as needed especially if orthopedic causes are suspected.
4. Electromyography and nerve conduction studies are helpful when suspecting a neuropathic or myopathic problem.
5. Videonystagmography and other vestibular and otologic tests may be helpful when a vestibular dysfunction is suspected as these tests may help distinguish central from peripheral vestibular disorders.
6. Comprehensive gait and balance analysis using instrumentation with optoelectronic systems, quantitative posturography, and shoe-integrated wireless sensor systems is possible but not widely available. Their role in the evaluation of most patients with gait difficulties is not well established at this point.
DIFFERENTIAL DIAGNOSIS
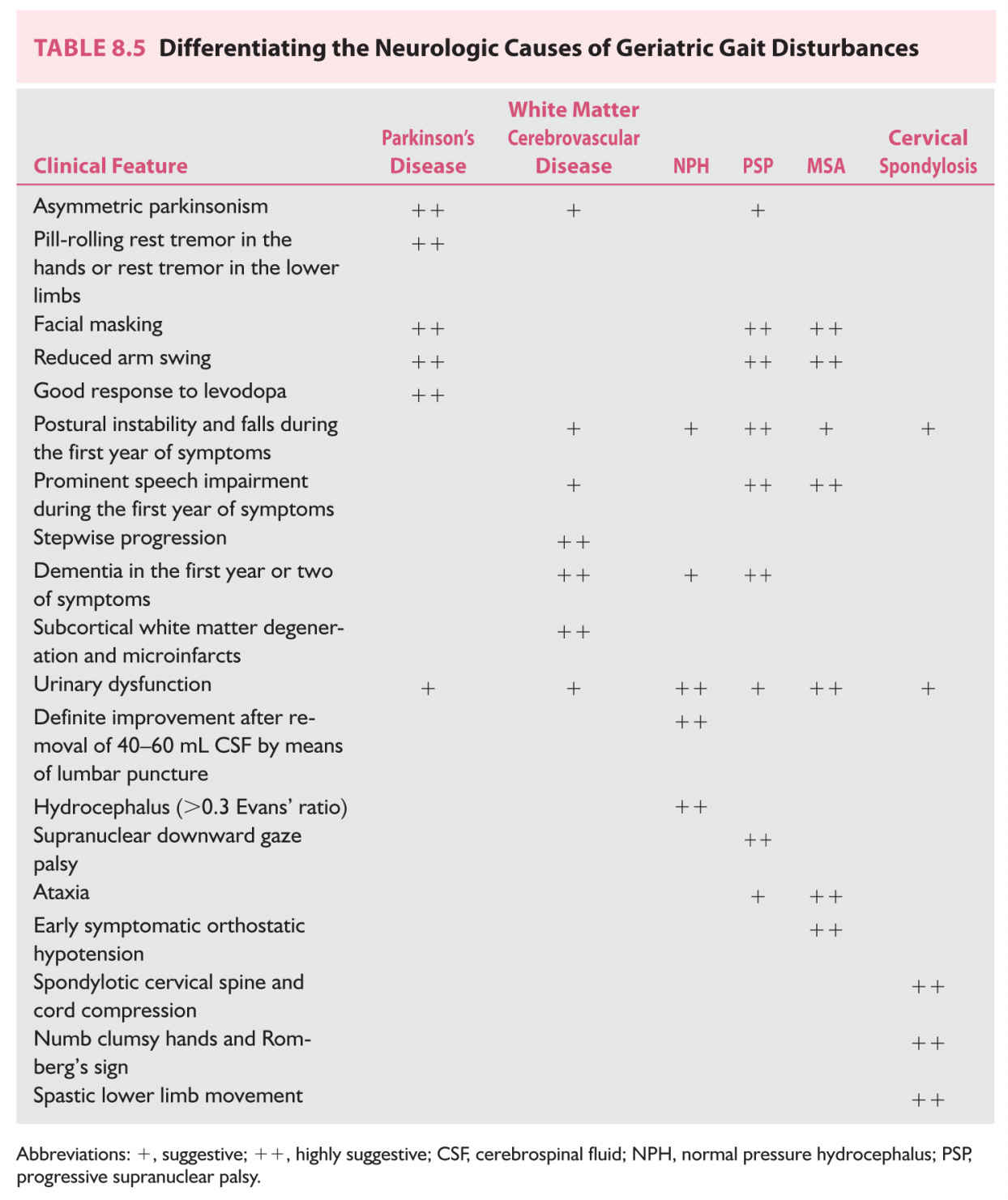
Differential diagnosis would depend on the gait patter observed and detailed history and physical exam. In older adults with fairly symmetric abnormal gait patterns it can be difficult to establish the origin of the gait and balance problem because of a significant overlap of symptoms. Some of the conditions with fairly symmetric gait difficulties seen in older adults are shown in Table 8.5.
DIAGNOSTIC APPROACH
The diagnostic approach is based on trying to identify the primary cause or causes of gait disturbance and all contributing comorbidities. Most of the gait abnormalities seen in older patients are multifactorial and some of these comorbidities can be easily overlooked as individually their relative weight in causing gait difficulties might be low. However, multiple, and apparently minimal, problems can add up and significantly contribute to walking and balance difficulties. Some examples of comorbidities worth exploring include vitamin B12 deficiency, arthritic limbs, spinal deformities, neuropathy, deconditioning, hypothyroidism, depression, foot disorders, cardiopulmonary disease, orthostatic hypotension, visual impairment, vertigo, and medications (e.g., sedative-hypnotics, anticonvulsants, antipsychotics, and antiemetics). These contributing conditions are frequently more treatable than the primary neurologic illness.
Criteria for diagnosis of gait disorders are heavily based on the medical and surgical history and findings on physical exam.
A. The diagnoses of neurodegenerative diseases (e.g., Parkinson’s disease, MSA, and progressive supranuclear palsy [PSP]) are based largely or entirely on the history and physical examination.
B. The diagnoses of other central and peripheral causes of gait disturbance are corroborated with neuroimaging or electrophysiologic studies, as needed.
C. The diagnosis of idiopathic NPH is particularly challenging as the symptoms are not specific and the classic clinical triad of gait disturbance, urinary incontinence, and cognitive dysfunction may also occur in patients with vascular dementia, chronic subdural hematoma, and degenerative dementia. In addition, enlarged ventricles can be seen in patients with cerebral atrophy and dementia (i.e., “hydrocephalus ex-vacuo”) that may already display some of those symptoms. Unfortunately, there is no ideal test to predict clinical response to CSF shunting.
1. Unequivocal improvement in gait after removal of 40 to 60 mL of CSF through a lumbar puncture supports the diagnosis but does not occur in all patients as it has a low sensitivity, between 30% and 60%. Improvement after external lumbar CSF drainage for 3 days is more sensitive (over 80%) and specific, but has increased risk of complications.
2. Improvement is achieved by approximately 50% of patients and sustained improvement by 30%. Complications occur in 20% of cases. Patients with an identifiable cause of hydrocephalus (e.g., aqueductal stenosis, Chiari’s malformation, and previous meningitis or subarachnoid hemorrhage) are more likely to respond than are those with idiopathic hydrocephalus.
3. Radiologic evidence of ventricular enlargement is necessary to establish the diagnosis of NPH. The Evans’ index has been used to establish evidence of ventriculomegaly. The Evans’ index is the ratio at the maximal frontal horn ventricular width divided by the inner diameter of the skull in axial cuts; a ratio >0.3 indicates ventriculomegaly. Unfortunately the index does not guarantee a beneficial response to ventriculoperitoneal shunting of CSF. As such the Evans’ index has little role to play in the decision to shunt CSF in patients with suspected NPH.
D. White matter brain lesions have been linked to impaired balance, slower gait, and reduced mobility. White matter hyperintensities seen on T2-weighted MRI images in the frontal lobe and periventricular regions show the strongest relationships with balance and gait difficulties; in particular, bilateral symmetric periventricular white matter lesions in the frontal and occipito-parietal regions have been found to be sensitive (frontal) or specific (occipito-parietal) in discriminating individuals with increased difficulties with their mobility. These white matter lesions can be measures through MRI imaging by using the Fazekas scale. This scale quantifies the amount of white matter T2 hyperintensities, usually attributed to chronic small vessel ischemia, and gives them a grade, from 0 to 3, based on size and degree of confluence of white matter lesions. Moderate and severe white matter lesions according to the Fazekas scale have been, on average, independently associated with a deterioration of gait and balance.
REFERRAL
A. Neurologic consultation. All older adults should be asked about falls at least once a year. If there is an abnormal gait or recurrent falls they will require further evaluation by a clinician with appropriate skills and experience.
1. A second opinion is advisable before shunting a patient with presumed NPH and before operating on a patient with presumed cervical spondylosis.
2. Drug-resistant parkinsonism is strong evidence against the diagnosis of idiopathic Parkinson’s disease.
B. Physical therapy and vestibular rehabilitation are considered cornerstones of management of gait and balance disorders. Vestibular rehabilitation is indicated when unsteadiness and vestibular dysfunction are involved and aimed at promoting vestibular adaptation and compensation. Prevention of falls should focus on physical conditioning and encourage regular physical activity. It should ideally be delivered through a multidimensional physical therapy activity program tailored to the patient’s needs; it should include exercises to enhance strength, endurance, balance, and flexibility. A comprehensive safety evaluation of the patient’s home by a physical therapist or visiting nurse is appropriate to establish potential fall hazards and suggest adaptations to the home environment. These adaptations may include the installation of handrails, raised toilet seats, grab bars in the shower, adequate lighting, and rubber floor mats among others. Elimination of throw rugs, clutter, and uneven surfaces would also decrease the risk of falls. The use of appropriately fitting shoes, with relatively firm slip-resistance soles and low heels is recommended and shoes with slippery soles, high heels, sleepers, and flip-flops should be avoided. Regarding mobility devices such as canes and walkers may improve balance and mobility if properly fit and should be prescribed after a complete evaluation by a physical therapist.
C. Referral to a podiatrist, orthopedist, or rheumatologist should be considered when skeletal or foot abnormalities contribute or cause significant walking difficulties.
Key Points
• Gait and balance disorders are common, especially among older adults, and a significant source of disability and limited quality of life.
• An active lifestyle is an important part of minimizing the risk of falls and balance difficulties as inactivity leads to deconditioning.
• Walking is a complex motor behavior that can be affected by lesions at multiple levels of the neuraxis and musculoskeletal system.
• A gait problem is the result of the primary gait difficulty and the associated compensatory mechanism.
• A gait pattern can help establish what structures of the neuraxis are affected and therefore localize the lesion; some gait disorders are very stereotypic and easy to identify (i.e., low- and mid-level gait disorders) while others are very variable and can be very challenging to diagnose and manage (i.e., HLGDs).
• Good proprioception, vision, and vestibular inputs are needed to maintain good balance; impairment of at least two of these inputs can seriously affect balance and this is the basis of the Romberg test.
• Central and peripheral vestibulopathies can cause balance difficulties but distinguishing both can be challenging at times; in peripheral vestibulopathy, all the signs are ipsilateral except for the fast component of the nystagmus. Lack of long-tract signs is not by itself indicative of a peripheral etiology. The head thrust test if performed properly can distinguish both (i.e., it is abnormal in peripheral vestibulopathies).
• Extensive white matter abnormalities seen on T2-weighted images and fluid attenuation inversion recovery (FLAIR)–weighted images on MRI, especially bilateral frontal or occipito-parietal periventricular ones, are associated with poor balance and walking difficulties.
• Defective cognition, in particular executive dysfunction, is linked to worsened performance while walking as shown in dual-task paradigms (e.g., more difficulty walking when asked to recite months of the year backward).
• There is significant overlap in the walking pattern of NPH and Parkinson’s disease. However, a way to help distinguishing both is that Parkinson patients can be very responsive to external cueing while NPH patients are not.
• There is no good algorithm to clearly diagnose and treat patient with NPH; a robust response to removal of 40 to 60 mL of CSF is very encouraging but the sensitivity of this test is very low in predicting response to CSF drainage. Trying an external lumbar drain for a few days is more sensitive but more prone to complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree