(Video 10.1). As the term “relative” explicitly states, an RAPD refers to a defect in the pupillary reaction to light of one eye relative to the other, and therefore bilateral RAPDs cannot exist. If vision loss in one eye has resulted in complete blindness, the direct reaction to light in that eye will be absent, known as an amaurotic pupil.
D. Confrontation visual fields should be performed at a comfortable distance from the patient with the examiner’s head at the same level as the patient’s. The patient covers one eye with their hand and is instructed to fixate on the examiner’s nose without looking at the examiner’s fingers. The examiner positions their head such that their nose is in the center of the patient’s visual field. The examiner presents one, two, or five fingers in each of the four quadrants of vision. Slowness to respond or an incorrect response in one quadrant or hemifield may be an early sign of homonymous visual field loss. If the patient is able to count fingers in each quadrant, fingers can be presented in any two adjacent quadrants simultaneously while asking the patient to compare the brightness and clarity of the fingers on each hand. Decreased brightness or clarity of one set of fingers may help increase the sensitivity of the examination and detection of a subtle hemianopia (suggestive of a chiasmal or retrochiasmal defect) or altitudinal visual field defect (may be seen with optic nerve or retinal disorders, such as anterior ischemic optic neuropathy [AION] or a branch retinal artery occlusion[BRAO]).
E. Ophthalmoscopy is typically performed with a handheld direct ophthalmoscope by most neurologists, which affords a magnified view of the posterior pole of the eye and facilitates a close view of the optic disc and retinal vessels. Recognition of abnormalities of the appearance of the optic disc is a key to the diagnosis of diseases affecting the anterior visual pathways. Pupillary dilation may be performed with 2.5% phenylephrine and 1% tropicamide. Details worth noting include the cup-to-disc ratio, appearance of the retinal vessels including spontaneous venous pulsations, the presence of hemorrhages or exudates, swelling or pallor of the optic discs, appearance of the macula, and the foveal light reflex.
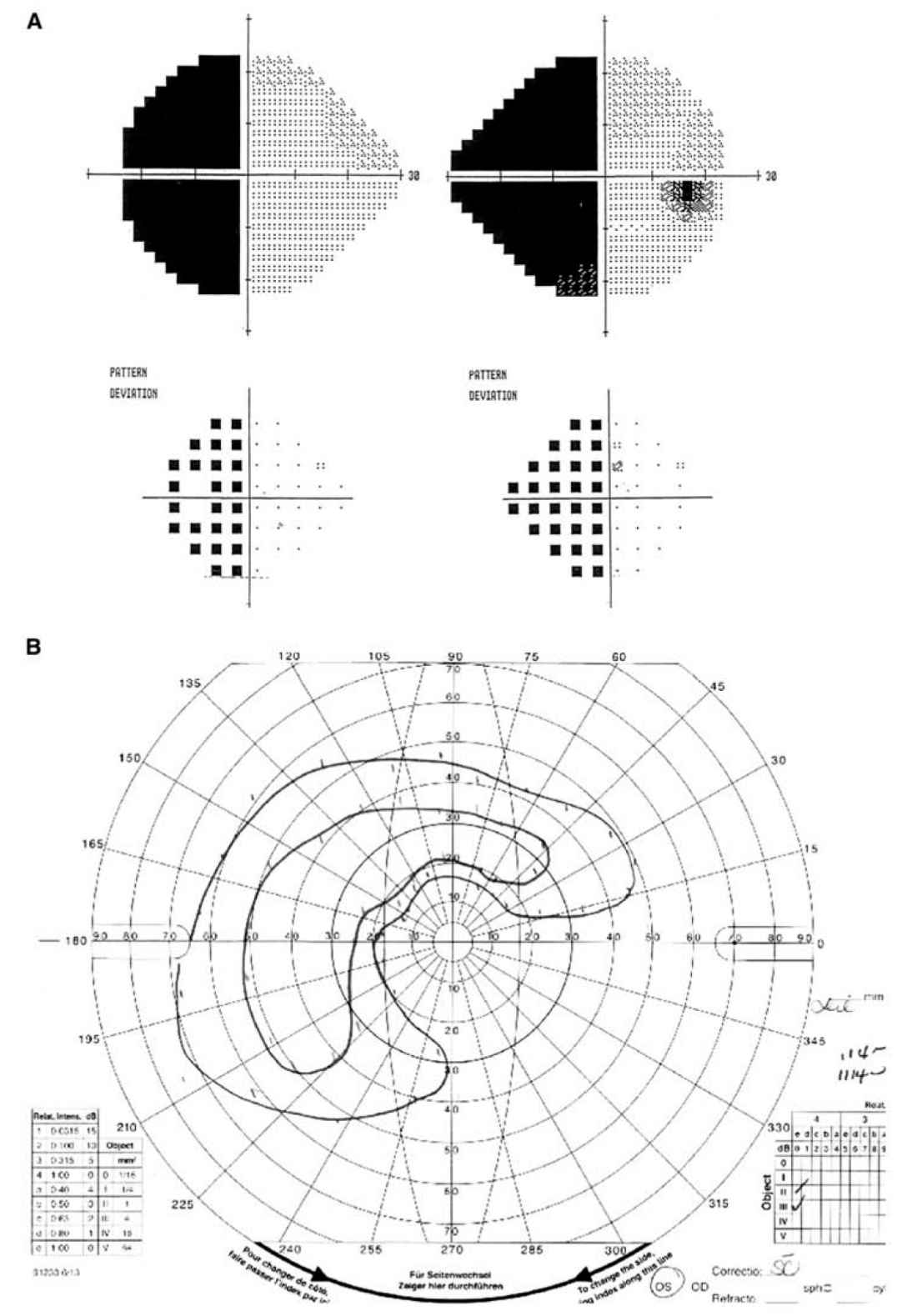
FIGURE 10.1 Visual fields. A: Humphrey visual fields showing a complete left HH. B: Goldmann visual field of the left eye showing a temporal and superior island of vision with vision loss centrally and inferonasally. The nasal field is to the right and the temporal field is to the left. HH, homonymous hemianopia. (See color plates.)
F. Visual field testing.
1. Static perimetry. Humphrey (Fig. 10.1A) and Octopus perimetry uses static targets to test the central 24 to 30 degrees of the visual field. The test is performed in many ophthalmology offices and is the most common form of perimetry performed. The test is sensitive, well validated, and capable of quantifying a visual field abnormality and following the defect over time.
2. Kinetic perimetry. Goldmann perimetry (Fig. 10.1B) uses moving lights of variable size and brightness to map the visual field using a kinetic technique. It is performed in an ophthalmologist’s office, but technicians trained in this method are becoming increasingly difficult to find. It is technician-dependent, and has largely been supplanted by static perimetry. It is less sensitive than automated static perimetry, but can be useful in patients who perform automated perimetry poorly, have severe visual loss, or have a suspected visual field defect outside the central 30 degrees of vision.
3. Tangent screen examination is rarely used today. It can be performed on a black felt screen located 1 m from the patient. A light is shone on the screen by the examiner from behind the patient and the visual field can be mapped. It can be especially helpful in screening for nonorganic “tunnel” vision. Patients with organic visual loss experience enlargement of the visual field when farther away from the screen. However, patients with nonorganic visual loss may insist the visual field size remains the same even with increasing distance from the screen.
G. Neuroimaging.
1. Computed tomography (CT)/magnetic resonance imaging (MRI). Imaging studies are often an essential part of the evaluation of a patient with vision loss, and determination of what area to image and the most useful protocol depends on the localization determined by the history and examination. An MRI of the orbits with and without contrast is the preferred neuroimaging study for monocular vision loss suspected to localize to the optic nerve. In cases of possible optic neuritis, imaging should also include the brain to assess the risk of multiple sclerosis. For bitemporal vision loss, even if asymmetric, perform imaging of the optic chiasm and sella with contrast, as in an MRI of the sella/pituitary. An MRI of the brain, with and without contrast, is best for the evaluation of the retrochiasmal visual pathways. CT has the advantages of lower cost and faster image acquisition time, but exposes the patient to radiation and provides inadequate detail of soft tissues that may be affected with vision loss, such as the optic nerves and brain parenchyma. CT can still be very useful in patients who have a contraindication to MRI.
H. Electrophysiologic testing.
1. Visual evoked potentials (VEPs). This test can be helpful in selected clinical situations, particularly in cases of suspected prior optic neuritis or suspected nonorganic visual loss. In patients with normal visual acuity and no color visual loss, a prolonged P100 latency may be seen in patients with a previous history of optic neuritis, which reflects subtle incomplete recovery of myelination of the anterior visual pathway. In patients with suspected nonorganic visual loss, a normal VEP waveform and latency can confirm normally functioning intracranial visual pathways. However, patients can focus in the distance, past the VEP fixation target stimulus, and alter the test results. Therefore, a normal VEP in the setting of suspected nonorganic visual loss is very useful, while an abnormal VEP may be inconclusive.
2. Electroretinography (ERG) is a test of electrical potentials generated by retinal photoreceptor function. A full-field ERG generates one waveform from the pooled response of photoreceptors from each eye separately, while a multifocal ERG generates multiple waveforms from small groups of adjacent cells in the retina and can detect more localized disturbances of retinal function. ERG is most commonly used to detect conditions such as retinitis pigmentosa and retinal dystrophies. Much like VEPs, a normal result may be useful in supporting a diagnosis of nonorganic visual loss when other structural and functional visual pathway testing is normal.
ACUTE TRANSIENT MONOCULAR VISUAL LOSS
A. Clinical features. Acute transient monocular visual loss (TMVL) has a variety of potential causes. TMVL from a vascular cause (retinal transient ischemic attack) is often referred to as “amaurosis fugax.” Patients may have difficulty distinguishing between truly monocular visual loss and a binocular homonymous hemifield defect, particularly if the visual loss event is short-lived or followed by a headache. The history is most likely to be reliable in patients who covered one eye during the event to determine whether one or both eyes were involved. Monocular visual loss may be reported as being sudden in onset, when in reality it was suddenly discovered when the unaffected normal eye was covered.
B. Time course. Distinction among the various causes of TMVL can be achieved to some degree with careful consideration of the time course of symptoms. TMVL occurring primarily with positional changes lasting several seconds is most suggestive of transient visual obscurations (TVOs) that occur with increased optic nerve head pressure in susceptible patients, such as those with papilledema (most common), an anomalous and crowded optic nerve head, or optic nerve head drusen. Visual loss over a period of minutes is more likely to be associated with a vascular cause. Common causes of true TMVL include retinal emboli (artery-to-artery emboli or cardiac emboli) and retinal/ocular hypoperfusion. Young patients with frequent TMVL and a negative workup may have vasospasm of a retinal artery. A loss of vision for minutes to hours involving a homonymous portion of the vision in both eyes may be a sign of cerebral transient ischemic attack or migraine.
C. Approach to TMVL.
Patients not yet examined by an eye care provider should undergo an eye examination with measurement of best-corrected acuity and intraocular pressure, dilated funduscopic examination, and visual field testing. Findings and testing that may be helpful in narrowing the diagnostic possibilities include:
1. Ophthalmoscopic evidence of asymmetric optic disc cupping (intermittent angle-closure glaucoma), optic disc anomalies (crowded, drusen, and so on), evidence of retinal embolism (hemorrhages, exudates, cholesterol emboli [Hollenhorst plaque], platelet, fibrin, or calcium emboli), and retinal hypotensive retinopathy (retinal hemorrhages with dilation of the veins).
2. An RAPD (Marcus Gunn pupil), visual field loss in both eyes, loss of visual acuity, and color vision deficiency all help localize a possible vision abnormality. TMVL with exercise or exposure to heat may occur in patients with a history of demyelinating or other forms of optic neuropathy (Uhthoff’s phenomenon).
3. Proptosis is a sign of intraorbital disease and can be associated with intermittent visual loss due to vascular or optic nerve compression, especially with eye movement in certain directions (gaze-evoked amaurosis).
4. Cardiac murmurs or carotid bruits.
5. Laboratory studies that may be helpful in the evaluation of TMVL include:
a. Complete blood count (CBC), including platelet count
b. Erythrocyte sedimentation rate (ESR)—in all patients over age 50
c. C-reactive protein (CRP)—in all patients over age 50.
6. Cervical vascular imaging is important as vascular TMVL may reveal ipsilateral carotid disease or aortic arch atheroma (depending on local availability, carotid Doppler ultrasound and/or transcranial Doppler, MR angiography [MRA], or CT angiography may be performed).
7. Brain imaging (ideally brain MRI with diffusion-weighted imaging) is systematically obtained acutely, looking for concomitant acute cerebral ischemia (in which case a brain MRI and MRA of the head and neck are usually performed).
8. Cardiac evaluation typically includes a transthoracic echocardiogram with agitated saline bubble study or transesophageal echocardiogram with careful examination of the aortic arch, left atrial appendage, and interatrial septum looking for a patent foramen ovale, atrial septal defect, and/or atrial septal aneurysm.
9. In patients with a negative vascular and cardiac workup, evaluation for a hypercoagulable state should be considered.
PERSISTENT MONOCULAR VISUAL LOSS
Monocular visual loss can localize anywhere from the spectacles a patient is wearing (or not wearing) to the optic nerve. Vision loss involving the optic chiasm or retrochiasmal visual pathways involves both eyes. The neurologist must be familiar with the clinical findings that help distinguish an optic neuropathy from ophthalmic causes of visual loss. Optic neuropathies are characterized by a loss of central visual acuity, decreased color vision often out of proportion to acuity, an RAPD (when vision loss is unilateral or asymmetric), and often an abnormal optic disc appearance such as pallor or edema. Exceptions exist, such as AION, which often spares central visual acuity, or typical retrobulbar optic neuritis in which the funduscopic appearance is normal during the acute phase. Nevertheless, the presence of an RAPD is one of the most reliable indicators of unilateral or asymmetric optic nerve dysfunction.
A. Acute/subacute clinical syndromes involving the optic nerve.
1. Optic neuritis. Inflammation of the optic nerve occurs most often in younger patients and presents with monocular visual loss and is associated with pain with eye movements in >90% of cases. The visual loss is most often central or altitudinal, but may involve any portion of the visual field. Patients may report colors as dim or “washed out.” Two-thirds of patients have a normal optic disc appearance acutely, while one-third have optic disc edema. Visual acuity can range from 20/20 to NLP, but most often is between 20/50 and 20/200. Visual recovery occurs over weeks and the prognosis for visual recovery is very good. Poor recovery of vision following an episode of suspected optic neuritis should prompt consideration of neuromyelitis optica, or a vascular event, such as ischemic optic neuropathy.
2. AION. AION typically presents in older patients with acute, monocular visual loss, which may worsen over several days. Acutely, all patients should have optic disc edema (Fig. 10.2A) and an RAPD (if unilateral and no prior contralateral optic nerve damage) (Video 10.1). There are two forms of AION:
a. Nonarteritic AION (NAION). Approximately 90% of AION cases are nonarteritic and one of the most important risk factors is a structural predisposition from a small, nearly cupless, optic disc (Fig. 10.2B). This “disc at risk” is predisposed to an ischemic cascade in which ischemia leads to optic disc swelling, which causes compression of fibers in the already crowded optic nerve head, leading to a decrease in optic nerve head blood flow that further increases ischemic damage and swelling, and so on. NAION is typically painless and characteristically associated with an altitudinal, nasal quadrantic, or central scotoma visual field defect. Patients with a history of NAION are at increased risk for involvement of the second eye. While some providers offer a course of oral steroids in selected cases, no treatment has yet been proven effective in the treatment of NAION.
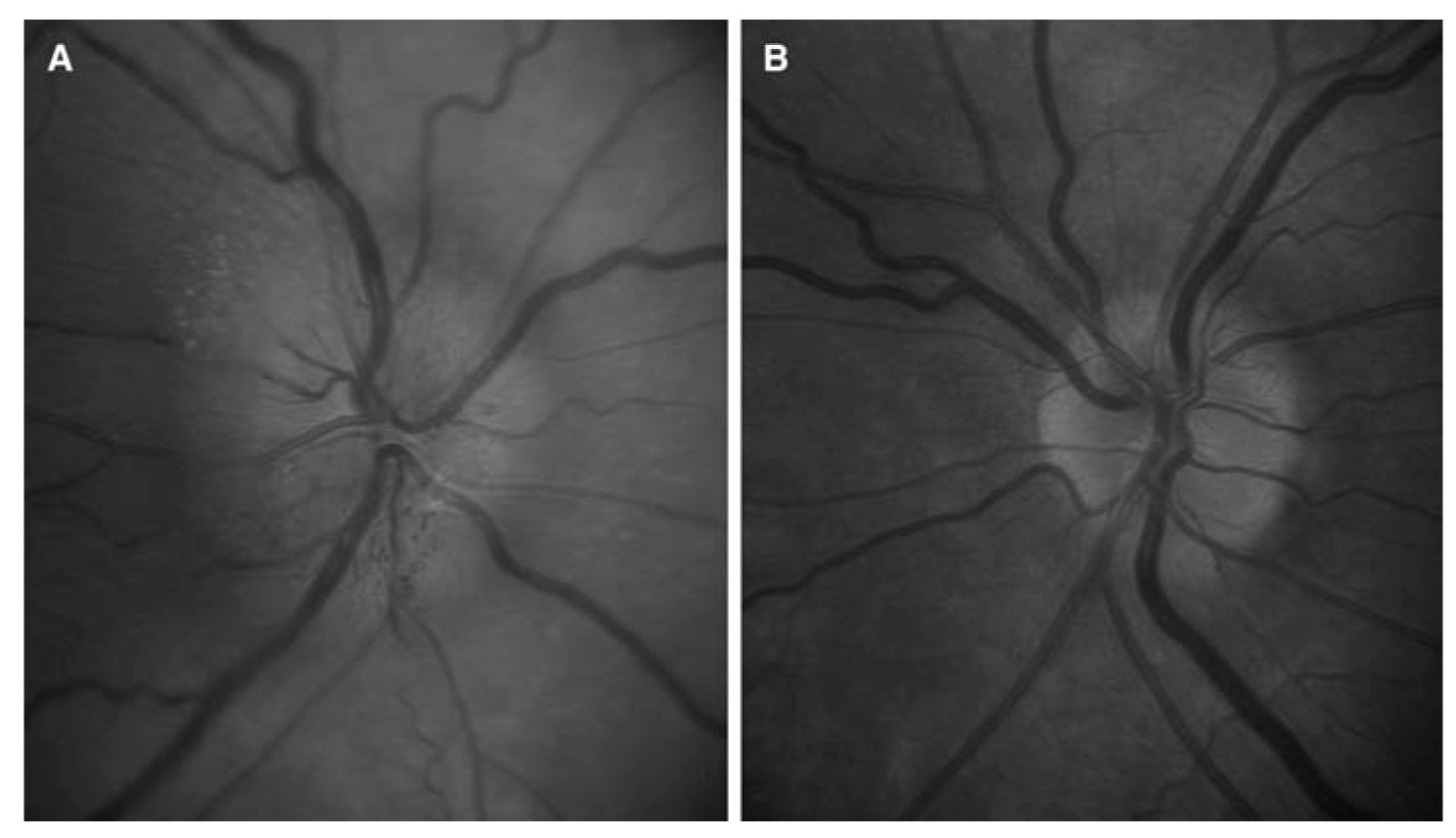
FIGURE 10.2 A: NAION showing acute optic disc edema in the right eye, with small peripapillary hemorrhages and exudates, as well as optic disc hyperemia. B: The optic disc in the patient’s fellow eye is small and cupless, known as a “disc at risk” for NAION. NAION, nonarteritic anterior ischemic optic neuropathy. (See color plates.)
b. Arteritic AION (AAION). AAION tends to occur in older individuals than those with NAION, and giant cell arteritis (GCA) is the most common cause. Symptoms suggestive of GCA include a new headache, scalp tenderness, preceding episodes of TMVL, and jaw claudication. Polymyalgia rheumatica may also be present. AAION typically causes sudden, severe, permanent visual loss. Visual acuity is usually 20/200 or less and visual field defects range from an altitudinal visual field defect to a large central scotoma or even complete blindness. The optic disc is always swollen acutely, and is often also pale (“pallid edema”). ESR, CRP, and CBC should be checked emergently and if the clinical history and laboratory results are suggestive, intravenous high-dose corticosteroids should be started without delay. A temporal artery biopsy should be performed, but treatment of suspected GCA should not be delayed under any circumstances. The prognosis for visual recovery is poor. The risk of involvement of the second eye in GCA without adequate treatment is very high, and may occur within hours or days.
3. Leber’s hereditary optic neuropathy (LHON) is a disorder of mitochondrial DNA, inherited through the maternal lineage. It presents as subacute, painless, monocular visual loss over days, and most commonly affects men in their teens to twenties (8:1 men to women). The second eye becomes similarly affected weeks to months after the first eye. During the acute phase, the optic disc may appear slightly swollen with a mildly hyperemic appearance (Fig. 10.3). After at least several weeks, the nerve fiber layer becomes atrophic, particularly in the region of the papillomacular bundle. Both optic discs become pale after nerve fibers are lost. Visual acuity typically ranges between 20/200 and 20/800 and there are dense central scotomas. Confirmation of the diagnosis is made with genetic testing screening for specific mutations in the mitochondrial DNA.
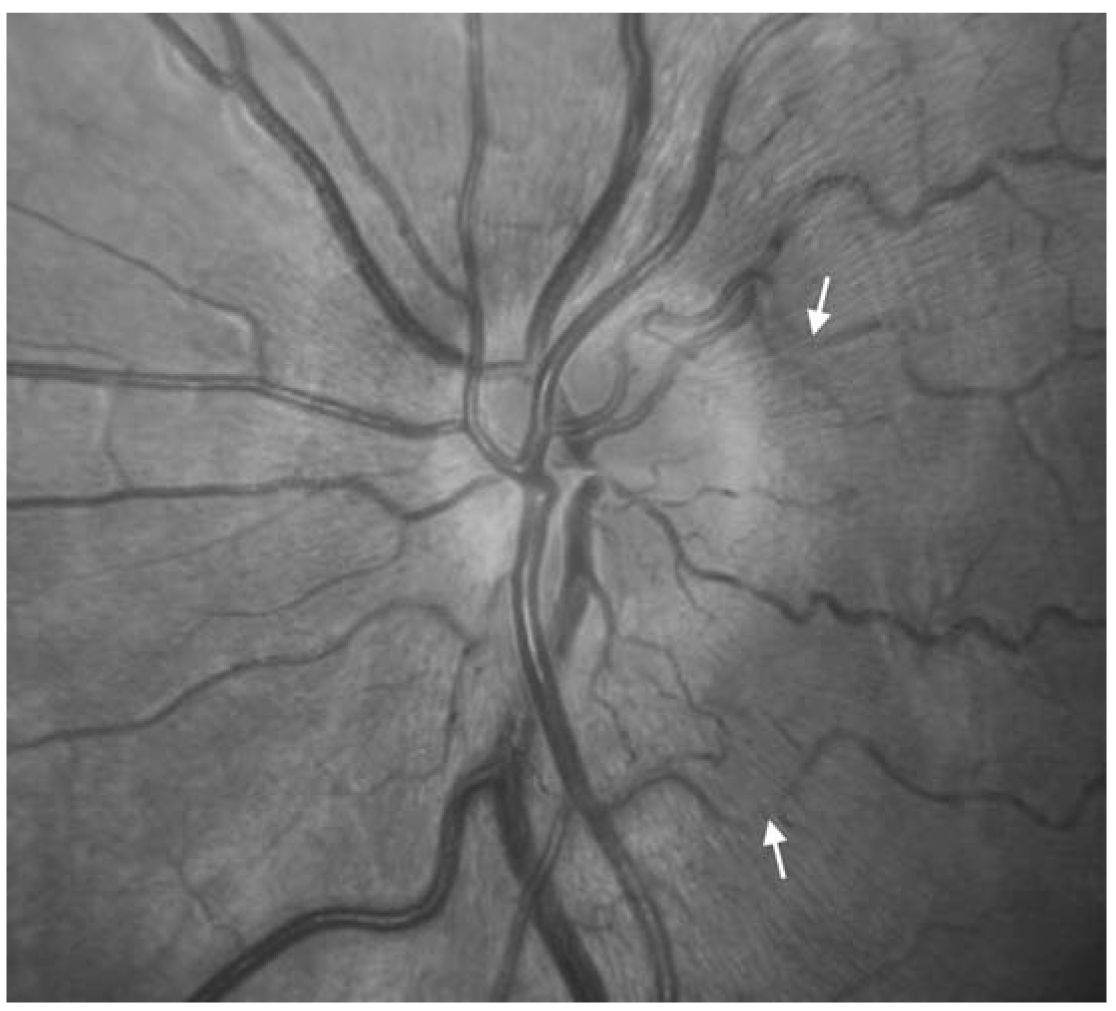
FIGURE 10.3 Acute LHON. There is thickening of the retinal nerve fiber layer (RNFL) surrounding the optic disc. The RNFL has a faintly white striated appearance and is oriented in a radial pattern from the optic disc. The thickened RNFL partially obscures some of the retinal vessels (white arrows). There is also hyperemia of the optic disc, with a “reddish” appearance. LHON, Leber’s hereditary optic neuropathy. (See color plates.)
B. Chronic monocular visual loss from optic neuropathy
1. Compressive optic neuropathies are typically painless and may slowly progress over years, or may progress more quickly, depending on the nature of the compressing lesion (Fig. 10.4). If the optic nerve is compressed by a mass within the orbit, there may be proptosis, limitation of extraocular motility, eye movement-induced transient visual loss (gaze-evoked amaurosis), conjunctival congestion, and chemosis. If optic nerve compression occurs within the optic canal or intracranially, proptosis occurs late, or may not occur at all. Clinically, there are features of optic neuropathy and the optic disc may appear normal (Fig. 10.4A), or exhibit optic disc edema, pallor, or both. Retino-choroidal collateral (shunt) vessels may develop on the optic disc in the setting of chronic disc swelling from optic nerve compression and impaired venous drainage.
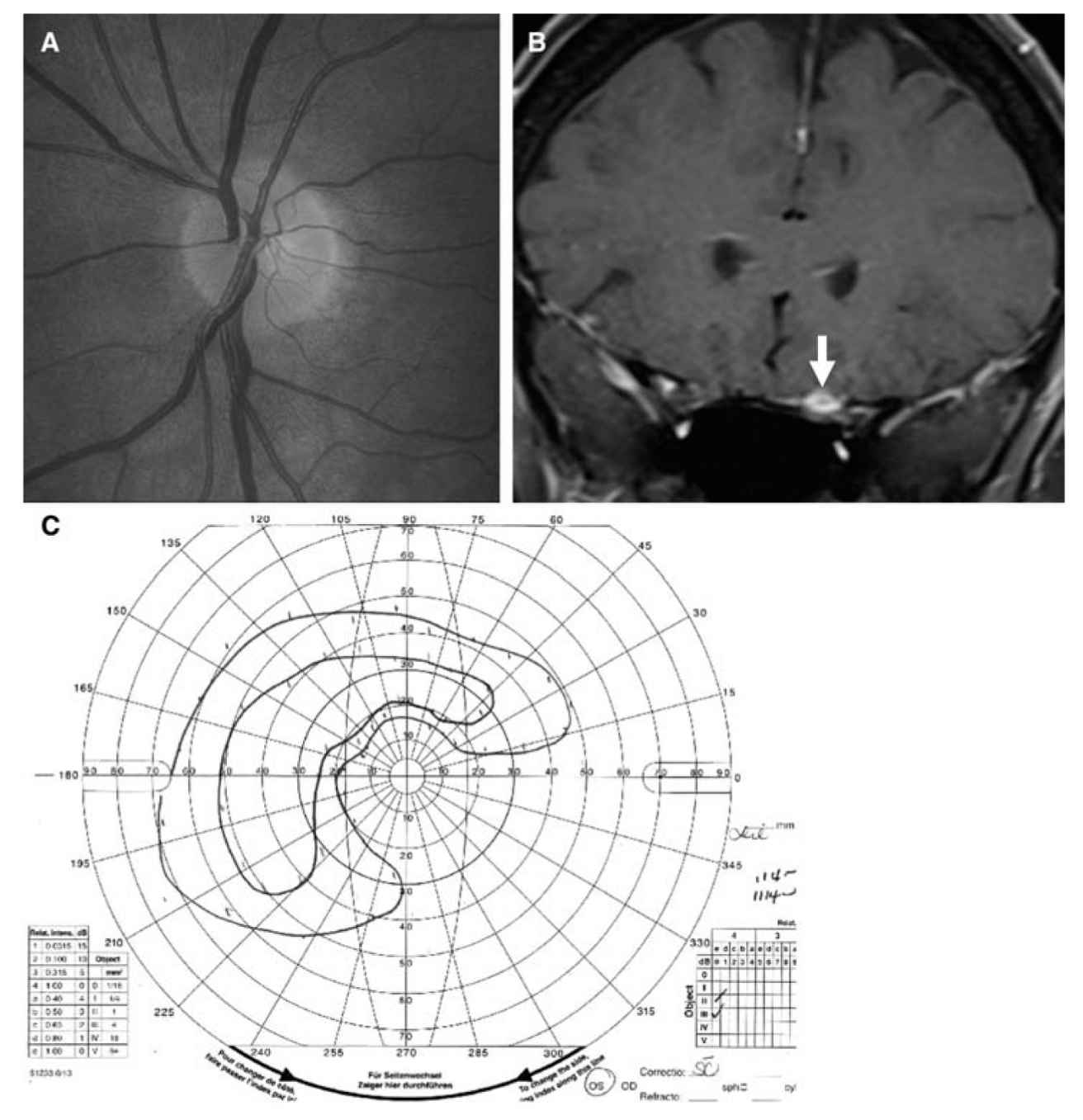
FIGURE 10.4 Planum sphenoidale meningioma causing a compressive optic neuropathy. A: Fundus photograph showing the patient’s normal-appearing left optic disc. B: Coronal postcontrast T1-weighted MRI showing enhancement of a mass encasing and compressing the left optic nerve (arrow), which was missed on a prior MRI performed without contrast. C: Goldmann visual field of the patient’s left eye showing a preserved island of vision superotemporally. (See color plates.)
2. Other causes of optic neuropathy include glaucoma (usually involves both eyes), dominant optic atrophy, toxic exposure (methanol, ethylene glycol, ethambutol), vitamin deficiency (vitamin B12), damage from papilledema or trauma, and inflammatory, infectious, and infiltrative optic neuropathies.
A. Acute onset binocular visual loss is most often because of chiasmal or retrochiasmal visual pathway lesions. Occasionally, acute binocular vision loss can be because of bilateral optic nerve dysfunction, such as ischemic optic neuropathy or optic neuritis (especially with neuromyelitis optica). On examination, one optic disc may be pale and atrophic, indicative of prior damage, and the other disc may show signs of acute damage, such as optic disc edema. This is characteristic of the “Foster Kennedy syndrome” in which a frontal tumor can cause an ipsilateral compressive optic neuropathy, as well as optic disc edema in the contralateral eye because of elevated intracranial pressure. A “pseudo-Foster Kennedy syndrome” can occur in conditions in which the optic disc edema is not because of papilledema. For example, prior AION in one eye may leave optic disc pallor, while acute AION in the fellow eye causes optic disc edema.
TVOs occur in patients with increased tissue pressure at the optic nerve head, which may be associated with papilledema, optic nerve head drusen, or an otherwise anomalous optic disc. TVOs are experienced as transient blurring or dimming of the vision, lasting for seconds, often precipitated by postural changes or Valsalva maneuver. Rarely, binocular visual loss may follow a cerebral or cardiovascular angiography procedure. A full recovery of vision is usually expected in hours to days, and permanent vision loss related to toxic contrast exposure is very rare.
BINOCULAR VISUAL LOSS DUE TO OPTIC CHIASM DYSFUNCTION
A. Clinical features
1. Bitemporal visual loss. Compression of the inferior portion of the optic chiasm, as in an expanding pituitary adenoma under the chiasm, results in superior bitemporal visual loss. Compression of the optic chiasm from above may occur with an aneurysm of the anterior cerebral artery or a craniopharyngioma and causes inferior bitemporal visual loss. Complete bitemporal defects are usually due to tumor compression of the chiasm, or may occur with a traumatic tear of the optic chiasm.
Some patients may not notice their bitemporal visual loss and may instead experience other symptoms related to instability of the two preserved nasal visual fields. These symptoms may include intermittent diplopia, loss of objects, and vertical sliding of one hemifield relative to the other. This “slip” in the retinas causes one-half of the image to slip vertically and/or horizontally in relation to the other half because of the brain’s difficulty in finding overlapping corresponding portions of intact visual field. These symptoms are known as “hemifield slide” phenomena.
2. Junctional syndrome. If dysfunction of the optic chiasm occurs at the point where one optic nerve meets the chiasm, a junctional syndrome occurs in which vision loss occurs centrally in that eye, and superotemporally in the fellow eye. In its most severe form, complete vision loss in one eye is accompanied by complete temporal hemifield loss in the fellow eye.
3. Pituitary apoplexy. An acute hemorrhage into a pituitary tumor or the pituitary gland (pituitary apoplexy) can cause the abrupt onset of unilateral or bilateral visual loss and may be accompanied by ocular motility disturbances related to dysfunction of cranial nerves III, IV, or VI. Headache, fever, and a stiff neck may be associated symptoms. Apoplexy may occur spontaneously or result from infarction following cardiac surgery or carotid endarterectomy. The diagnosis may be confirmed with an MRI or CT scan. Only rarely does pituitary apoplexy cause pure visual loss and is more often associated with some ocular motility disturbance. Pituitary apoplexy is a “don’t miss” diagnosis among the various causes of sudden bilateral visual loss.
B. Clinical approach to nonacute binocular visual loss
1. As with more acute visual loss, attempt to localize the deficit with the available history.
2. On examination, visual acuity, color vision, and confrontation visual fields should all be assessed.
3. Ophthalmoscopy may reveal important clues. For example, optic disc pallor is indicative of intracranial visual pathway injury, usually to the optic nerve, at least 4 to 6 weeks after an acute event. Hemorrhages and optic disc edema can be acute or chronic. Bowtie pallor, in which there is pallor of the temporal and nasal optic disc, sparing the superior and inferior disc, occurs when the fibers that cross at the optic chiasm (nasal optic disc and papillomacular bundle/temporal optic disc) are atrophic because of damage of the optic chiasm or pregeniculate retrochiasmal visual pathways.
HOMONYMOUS HEMIANOPIA
Homonymous hemianopia (HH) is a term used to describe hemifield loss that is on the same side of the vertical meridian in both eyes (Fig. 10.5). A HH can be complete or incomplete depending on the extent of hemifield involvement, and congruous or incongruous (in incomplete hemianopias), depending on how similar the visual field is in one eye compared with the other. Associated signs, such as hemiparesis, hemisensory loss, aphasia, or parietal neglect, also give clues to the localization. In general, the more posterior the visual pathway lesion in the post-geniculate visual pathways, the more congruous the visual field defect between the two eyes.
A. Localization. HH localizes to the retrochiasmal visual pathways on the side of the brain opposite the visual field defect, which include the optic tract, the lateral geniculate nucleus (LGN), optic radiations (geniculocalcarine tract), and occipital cortex.
1. Optic tract. A HH accompanied by an RAPD on the same side as the hemianopia is a sign of optic tract dysfunction.
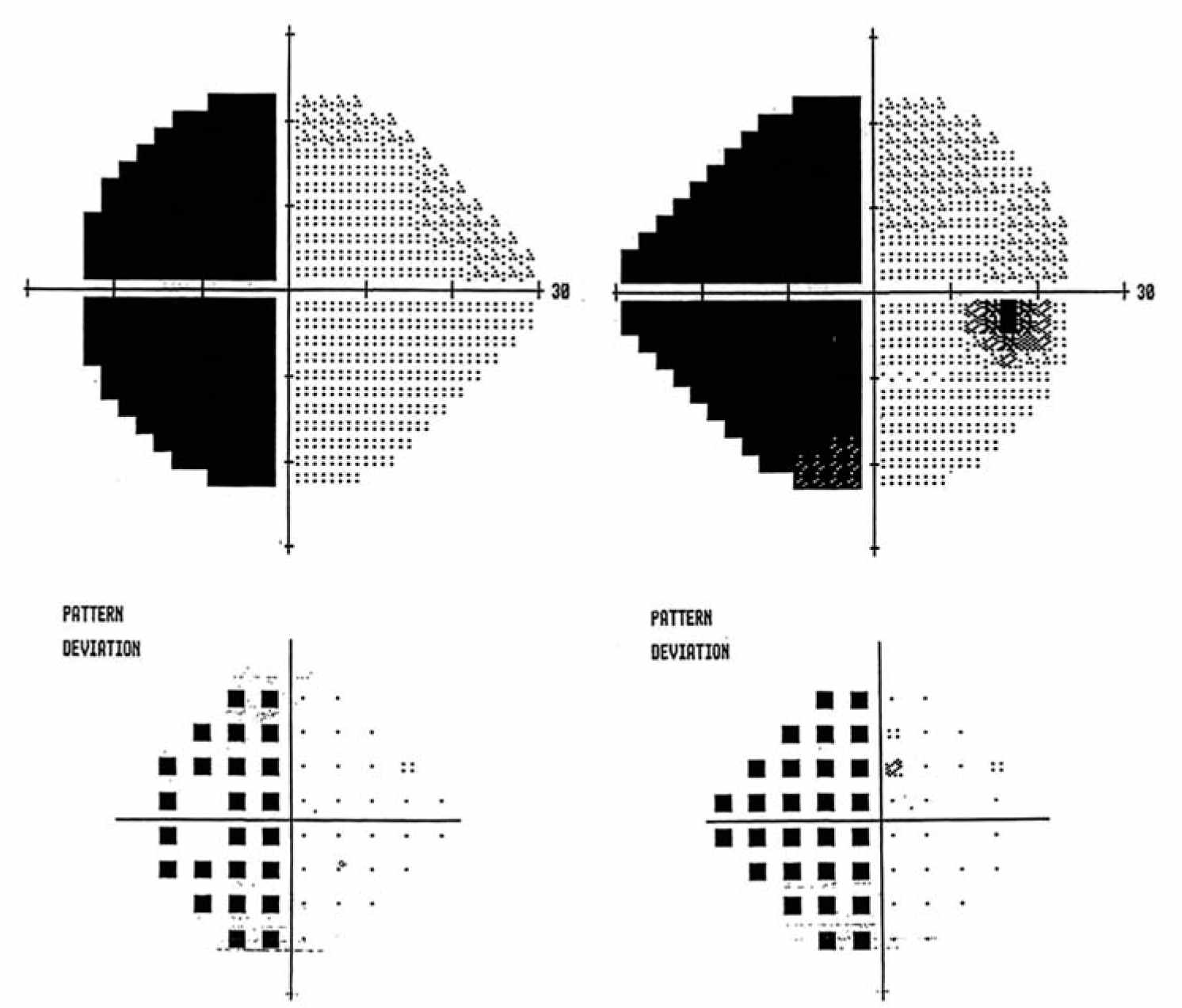
FIGURE 10.5 Homonymous hemianopia. Humphrey visual fields showing a complete left HH. HH, homonymous hemianopia.
2. LGN. The LGN has a dual blood supply from the lateral choroidal artery (a branch of the posterior cerebral artery) and the anterior choroidal artery (a branch of the internal carotid artery). Occlusion of the lateral choroidal artery causes a homonymous sectoranopia around the horizontal meridian, and occlusion of the anterior choroidal artery causes a HH sparing the sector around the horizontal meridian. Mass lesions of the LGN more often produce a complete HH.
3. Primary visual cortex. Occipital lobe damage produces pure visual loss if the damage is confined to the calcarine cortex (primary visual cortex). Total loss of the calcarine cortex on one side will give a complete HH. Commonly, the macular region of calcarine cortex is “spared” to some extent because a large part of the visual cortex closest to the occipital pole subserves the inner 20 degrees of the visual field. Also, there may be a dual blood supply to the occipital pole, with anastomoses from branches of the middle and posterior cerebral arteries. Small occipital pole infarcts that cause visual loss within the central 20 degrees of the visual field can be difficult to detect by standard perimetry.
B. Migraine aura. One of the most common causes of episodic homonymous visual loss is the visual aura of migraine, which some patients may mistakenly believe is associated with visual loss in only one eye (the eye with the temporal visual field defect). Characteristic features include positive visual phenomena such as colored lights that flicker or shimmer in a zig-zag pattern surrounding an area of visual loss (“scintillating scotoma”) that may expand over a period of minutes. Other descriptions include a “heat wave” like the appearance just above a road in the distance on a hot day, or a central visual image in both eyes “as if a flashbulb just went off.” These visual events usually last from 5 to 60 minutes and are often followed by a headache. The headache is classically unilateral, but may be generalized. Some patients, especially older migraineurs, may have only the visual aura without a headache, called acephalgic migraine.
C. Occipital lobe epilepsy. Seizures with primarily visual manifestations without secondary generalization may be seen in patients with metastatic brain tumors, meningiomas, gliomas, and arteriovenous malformations affecting the occipital lobes. The seizures may produce homonymous flickering lights, flashes, and colors, but unlike migraine, there is no characteristic buildup and progression of visual symptoms from the center to the periphery. A head CT or brain MRI usually reveals the responsible lesion and an electroencephalogram (EEG) may show epileptiform activity localized to the occipital region.
D. Degenerative diseases. The Heidenhain variant of sporadic Creutzfeldt–Jakob disease (CJD) may present with a rapidly progressive HH as the condition affects the occipital lobes out of proportion to other brain regions. Rarely, patients with other neurodegenerative diseases, such as Alzheimer’s disease (AD), may develop a HH. CJD can be distinguished from AD by the time course of symptom progression and characteristic MRI and EEG changes. Progressive multifocal leukoencephalopathy may present with a HH and demyelinating changes on MRI that characteristically spare the U-fibers.
E. Posterior reversible encephalopathy syndrome (PRES) can present with a HH in cases with asymmetric involvement of the occipital lobes, or cerebral blindness when there is bilateral involvement. PRES is likely related to cerebral edema from failure of the blood–brain barrier to appropriately compartmentalize intravascular fluid. PRES can be precipitated by hypertension, toxic medications, and various diseases.
VISION DISTURBANCES RELATED TO HIGHER COGNITIVE DYSFUNCTION
These visual disturbances are most commonly caused by embolic stroke, but may also be caused by primary or metastatic tumors, or other lesions. The unusual symptoms produced by lesions of the association visual areas in the brain, often with a lack of vision loss, frequently go unrecognized.
A. Alexia without agraphia is caused by damage to the connections between both visual cortices and the angular gyrus. This condition can be associated with a HH in the case of a left occipital infarct that also involves the splenium of the corpus callosum. Alexia without agraphia can also exist without a HH with lesions to the splenium of the corpus callosum (outflow from the right occipital lobe) and the connections of the left occipital lobe to the angular gyrus. In all cases, words “read” by the occipital lobes are not properly relayed to the appropriate language centers in the dominant hemisphere.
B. Balint’s syndrome is characterized by visual disorientation, optic apraxia (“spasm of fixation”), optic ataxia (defect in visually guided hand movements), and simultanagnosia (inability to put together pieces of a visual scene) due to bilateral damage to the border zone between the middle and posterior cerebral artery territories in the high parietal lobes. Affected patients may have difficulty articulating their visual disturbance, but are not typically demented or aphasic.
C. Bilateral inferior temporal lobe damage with damage to the V4 area of the fusiform gyrus may cause prosopagnosia (the inability to recognize faces) and central achromatopsia (central color vision loss).
1. Prosopagnosia is the inability to recognize individual faces, but patients may also have trouble recognizing their car, dog, or other items among similar items. They can identify classes of objects, but have difficulty singling out a specific individual within a general class or group without other clues. They may compensate by becoming increasingly adept at recognizing features of a person’s voice or the way they walk to help identify them. There may be a homonymous superior quadrantic visual field defect or no associated visual loss. Patients may have a sudden deficit from bilateral inferior temporal lobe damage or a previously damaged inferior temporal lobe and then a second lesion to the other inferior temporal lobe. Less commonly, a patient may have prosopagnosia with a unilateral right inferior temporal lobe lesion.
2. Central achromatopsia can involve one hemifield in both eyes (hemiachromatopsia) or the entire visual field. The degree of color loss may vary from profound achromatopsia to more subtle desaturation of colors. The responsible lesions are in the inferior temporal lobes.
D. Anton syndrome is caused by extensive bilateral damage to both the occipital and parietal lobes, causing visual loss and denial of blindness. Patients often confabulate elaborately in answering questions about their visual environment.
NONORGANIC VISUAL LOSS
Patients with nonorganic vision loss claim partial or total visual loss in one or both eyes despite normal eyes and intracranial visual pathways. The vision loss can be voluntary or involuntary. Severe vision loss in one eye, when related to an optic neuropathy, is always accompanied by an RAPD. The lack of an RAPD in the case of severe monocular vision loss may raise suspicion for nonorganic visual loss after other intraocular causes have been excluded by an ophthalmologist. Various examination techniques can be used to confirm nonorganic visual loss. An optokinetic flag or drum can screen for optokinetic nystagmus in each eye individually. The presence of an optokinetic response requires visual acuity of at least 20/400. A mirror can also be held in front of the patient’s face and then tilted up and down and side to side. The eyes will usually move to orient the patient in space or follow the patient’s own reflection. Moderate binocular nonorganic visual loss (e.g., acuity of 20/50 in both eyes) can be difficult to prove, as the lack of an RAPD and a normal optokinetic response offer no additional useful information. Electrophysiologic testing, such as a VEP or ERG, can be helpful. Patients can voluntarily alter these tests to give an abnormal response, so these tests are most helpful when the results are normal. Severe bilateral nonorganic visual loss can be inferred from failure of proprioception tests that appear to require vision, such as “bring the tips of your index fingers together” or “sign your name.” Having the patient make several hand signals that are both shown and described followed by one in which the sign is shown, but not described, can sometimes uncover nonorganic visual loss.
A. Approach to the patient with suspected nonorganic visual loss.
1. Observe the patient carefully, including how they entered the examination room, their response to visual cues, eye contact, and resistance to testing. Document each visual test performed and the patient’s response, including behavior.
2. Greater resistance to the examination may be seen in deliberate malingerers more often than naïve nonorganic patients who may be oblivious to obvious contradictions in their examination performance.
3. Once the examination is complete, confronting the patient is rarely helpful. Suggesting that the vision is “better than the patient is aware” and is likely to improve can be helpful. Malingerers may accuse the examiner of not believing their symptoms or suggesting that they are simply “all in their head.”
4. Radiographic (CT and MRI) and electrophysiologic studies (ERG and VEP) should be interpreted for the patient to be sure that no doubts regarding the etiology of vision loss remain. The patient should be told that there is no disease of the nervous system causing these symptoms. It may be useful to reassure them that “the structure and function of the eyes and the parts of the brain that control vision are working normally. In some people, the brain temporarily has trouble using the eyes and parts of the brain that control vision, even though they are normal.”
5. It can be counterproductive to hope for a placebo effect by prescribing unnecessary eye drops or a medication, which undermines the assertion that there is no disease causing the symptoms. Artificial tears are reasonable to recommend to patients with signs or symptoms of dry eyes.
B. General rules in the evaluation of patients with vision loss
1. Perform a history and examination with localization of the vision loss in mind.
2. Perform imaging studies specific to the localization determined by your history and examination. For example, for monocular vision loss suspected to localize to the optic nerve, perform an MRI of the orbits with and without contrast. In cases of possible optic neuritis, imaging should also include the brain. For bitemporal vision loss, even if asymmetric, perform imaging of the optic chiasm and sella with contrast, as in an MRI of the sella/pituitary.
3. An evaluation by an ophthalmologist should always be performed in cases of monocular or binocular vision loss that do not clearly localize to the optic nerves or intracranial pathways of vision. The ophthalmology evaluation can include automated visual field testing, fundus photography, and optical coherence tomography that may not be available in most neurology clinics.
Key Points
• In patients with visual loss, the history and examination should first focus on localization, which helps with forming an appropriate differential diagnosis and directing additional testing.
• In the absence of a severe unilateral retina problem (which should be easily visible to an ophthalmologist), an RAPD indicates an optic neuropathy.
• MRI of the orbits with contrast and fat suppression techniques is the imaging modality of choice in patients with a suspected optic neuropathy.
• Patients with visual loss not obviously ascribable to a lesion of the intracranial pathways of vision should always be examined by an ophthalmologist.
• Patients with nonorganic vision loss may still have an organic cause of vision loss, with conscious or subconscious embellishment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








