Artifacts of Recording
Barbara Dworetzky
Susan Herman
William O. Tatum IV
Recording electric activity from the brain is subject to extracerebral interference or “artifact” that is present in essentially every electroencephalogram (EEG) (1). Artifacts may be derived from a variety of sources. They can obscure the cortically generated signal to a variable degree and render it impossible to interpret or lead to misinterpretation of the EEG as abnormal and possibly “epileptic” in origin. Failure to recognize such potentials as artifact is one of the leading reasons for inappropriate use of antiepileptic medications, and a common reason for patient referral to an epilepsy center (2,3).
The ideal EEG reflects purely cerebral activity and negligible extracerebral electric activity. It is performed by a qualified EEG technologist who ideally creates an artifact-free recording within the confines of a technically adequate recording on a cooperative patient in a quiet and controlled environment. This ideal EEG is pragmatically unachievable but the principles behind such a recording form the basis for recognizing the “contaminants” or “noise” that exist in the locations where EEG is performed. EEG interpretation is an acquired visual art, and the identification of common artifacts is often a matter of “automatic” recognition by the reader.
To minimize confusion generated by artifact, approved guidelines for electrode placement are essential (4,5). With the ubiquitous use of digital EEG synchronized with video, newly discovered rhythmic artifacts are being identified on a regular basis in special care units (SCUs). Offline reformatting of the EEG is not only possible, but also desirable, placing the responsibility of recognition, identification, and elimination of EEG artifact on the physician interpreting the EEG as well as the technologist. The burgeoning field of epilepsy and ICU monitoring with video correlation has led to easy confirmation of artifact sources and has produced many “art(ifact) collectors.” Some artifacts are extremely common and desirable for routine recording purposes. For example, eye movements can help define the normal stages of sleep. The foundation for recording principles in EEG may be subdivided into artifacts that are physiologic and those that are nonphysiologic (Table 13.1). Physiologic artifacts include those generated by the patient’s biologic activity with electric potentials that are generated near the head (muscles, eyes, heart), or by patient movement. These may require camouflaging or ignoring the extracerebral source since elimination is not always possible. Nonphysiologic artifacts are generated anywhere in the EEG recording system from the electrode-scalp interface to the instrument itself or from the environment, including from devices near or within the patient. While many biologic artifacts show characteristic waveform morphologies and electric fields and therefore can be fairly easily identified, nonphysiologic artifacts show a wide variety of morphologies that can either distort or obscure normal EEG activity. In the most severe cases, artifact may render the recording uninterpretable. When artifacts result from equipment malfunction, the EEG machine should be immediately tested to ensure there are no electric safety hazards. In the digital age of EEG recording, it is easier to know with certainty the generators of EEG contaminants by examining the patient relative to the recording, and subsequently successfully eliminating the artifact that is identified (proof of principle). Illustrations of artifacts are provided to enhance the principles in this chapter.
GENERAL PRINCIPLES
The recognition of EEG artifact is an acquired skill. It relies on maintaining a low threshold to expect artifact in every epoch of the recording. For certain ubiquitous artifacts such as eye movements, noticing diminished amplitudes, slower frequencies, or changes in the direction of the dipoles, for example, may suggest a state change (i.e., drowsiness, rapid eye movement [REM] sleep). A marked asymmetry in eye movements may suggest asymmetric electrode placement, enucleation (prosthetic eye), or a frontal skull defect (6).
There are some general principles that can be applied when confronting possible EEG artifact. Artifact caused by loose electrodes can be distinguished from normal activity by its sometimes characteristic shape, positive charge, restriction to a single electrode, and apparent superimposition on normal cerebral
activity. Because the artifact is usually limited to a single electrode, it will be seen only in channels containing that electrode and will not show a “field” to neighboring electrodes. Reformatting into various montages, particularly referential montages, may help to identify a discharge as artifactual. Because some electrode artifacts may be difficult to recognize and may mimic abnormal patterns, any electrode showing high impedance or frequent pops should be reapplied or replaced. In some cases, high impedance or loose electrodes show severe artifact with minor patient movements. These electrodes should be reapplied as well. Confirming that electrodes are placed and labeled correctly in the proper headbox jack should be accomplished when expected waveforms appear in the wrong channel.
activity. Because the artifact is usually limited to a single electrode, it will be seen only in channels containing that electrode and will not show a “field” to neighboring electrodes. Reformatting into various montages, particularly referential montages, may help to identify a discharge as artifactual. Because some electrode artifacts may be difficult to recognize and may mimic abnormal patterns, any electrode showing high impedance or frequent pops should be reapplied or replaced. In some cases, high impedance or loose electrodes show severe artifact with minor patient movements. These electrodes should be reapplied as well. Confirming that electrodes are placed and labeled correctly in the proper headbox jack should be accomplished when expected waveforms appear in the wrong channel.
Table 13.1 EEG Artifact | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Neurosurgical procedures and certain developmental disorders disrupt normal cortical architecture and can yield positive discharges or ones with unusual fields (7) and thus mimic electrode artifact. Skull asymmetries and edema require documentation to properly reflect the differing amplitudes seen on the recording. Breach rhythms that emerge following craniotomy may become “spikier” with superimposition of myogenic potentials (Fig. 13.1). Rhythmic or periodic activity on an EEG recording should be matched with known rhythmic physiologic (i.e., cardiac, respirations) or nonphysiologic activity (i.e., drips, pumps, machines) to uncover the source. If the waveforms appear in more than one nonadjacent brain region at once (nonanatomic pattern), the activity is likely to be due to artifact. Additionally, if the waveforms appear in all leads and are unusual in appearance, the reference electrode, ground, or preamplifier should be checked for integrity.
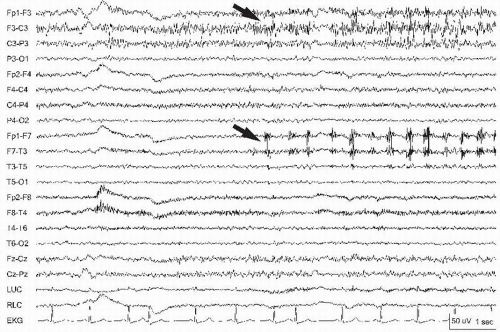 Figure 13.1 Myogenic potentials (arrows) superimposed on a breach rhythm in the left parasagittal chain can appear “spikier.” |
The elimination of artifact from the EEG once it is recognized involves identifying the source. Troubleshooting artifact involves checking the electrodes (including the ground and impedances), the apparatus, the patient (if still connected), and the ambient activity. Artifact, most apparent in a loose or dry electrode, is easily eliminated by regelling or replacing the electrode during the study. Eliminating the artifact by changing a lead or turning off an external device can confirm the source. The technologist should annotate the recording when activity generated by the patient or the external environment is interfering with the quality of the study. EEGs that run continuously over days such as epilepsy monitoring, ambulatory EEG, or ICU monitoring studies may contain large amounts of artifact. This may not allow proper artifact elimination as the technologist is not present for the majority of the recording. However, even when a technologist is on site, troubleshooting is not always simple or successful. It may be necessary to camouflage rather than eliminate artifact. Artifacts can mimic nearly any abnormal pattern (8), so it is critical that every attempt to discover the origin of the artifact be undertaken.
ARTIFACTS OF PHYSIOLOGIC ORIGIN
Physiologic sources of artifacts, apparent on every EEG recording, are generated from the biologic properties of the patient and the many sources within the body that can act as electric
dipoles. The most common and important of these artifacts are produced by the eyes, the muscles of the head and face, and the heart. Other important physiologic generators of artifact include the tongue or glossokinetic artifact, respiration, and potentials generated in the skin by sweat glands. In addition, the patient’s own movements, especially if rhythmic, may be a source of confusion on an EEG. Placing an extra electrode on or near a moving body part or utilizing video allows the reader to confirm the nonbrain origin.
dipoles. The most common and important of these artifacts are produced by the eyes, the muscles of the head and face, and the heart. Other important physiologic generators of artifact include the tongue or glossokinetic artifact, respiration, and potentials generated in the skin by sweat glands. In addition, the patient’s own movements, especially if rhythmic, may be a source of confusion on an EEG. Placing an extra electrode on or near a moving body part or utilizing video allows the reader to confirm the nonbrain origin.
Eye Movement Artifact
Eye movement artifacts are seen in virtually every awake and conscious individual during routine EEG. Most eyeball artifact is easy to recognize by its anterior location and its bilateral, synchronized appearance. When the eyes move, the electric dipole between the positively charged cornea and the negatively charged retina also moves, yielding a large potential depending on the direction and rate of the movement. In general, it is symmetric as long as lead placement is measured well, the patient is not missing an eyeball (Fig. 13.2), both retinas are functional, and the frontal bones are intact (6). Eye movement monitors placed above and below the eye using one to two channels help identify potentials that are in phase with cerebral, and out of phase with extracerebral, ocular sources (9). Eye movement artifact is important for the determination of the normal waking background as eye opening blocks the alpha rhythm and eye closure produces alpha. The artifact is produced by the Bell’s phenomenon as the eyeballs roll upward with the closure of the eyes, producing an upward movement of the positive dipole created by the cornea. Eye movements can be quite rapid with fluttering eyelids occurring at rates up to 13 Hz and appear abnormal (Fig. 13.3) especially if they are asymmetric (Fig. 13.4)! They can also be rather slow in the delta range mimicking abnormal patterns such as frontal intermittent rhythmic delta activity (FIRDA; Fig. 13.5). With horizontal eye movements, the artifact is generated over the lateral eye leads, F7 and F8 (Fig. 13.6). It can be quite striking as in the case of lateral gaze nystagmus, generally noted more from the side of the fast phase (10). The characteristic deflection that includes a rapid rise and more gradual fall with the corrective movement is quite easy to recognize. The side of the positive phase reversal of the rapid rise indicates the direction of the fast component of the nystagmus. With leftward eye movement, the F7 electrode has positive reversals at the same time
that the F8 electrode reveals negative reversals (Fig. 13.7). Additionally, eye movements are crucial to correctly identify the stages of sleep such as the slow roving subdelta in the lateral eye leads representing drowsy eyeballs during stage Ia and REM artifact also seen in F7 and F8 lateral eye leads during REM sleep (Fig. 13.8).
that the F8 electrode reveals negative reversals (Fig. 13.7). Additionally, eye movements are crucial to correctly identify the stages of sleep such as the slow roving subdelta in the lateral eye leads representing drowsy eyeballs during stage Ia and REM artifact also seen in F7 and F8 lateral eye leads during REM sleep (Fig. 13.8).
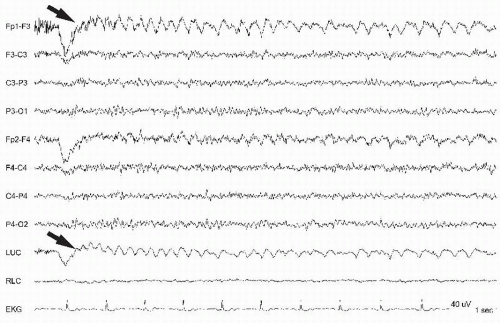
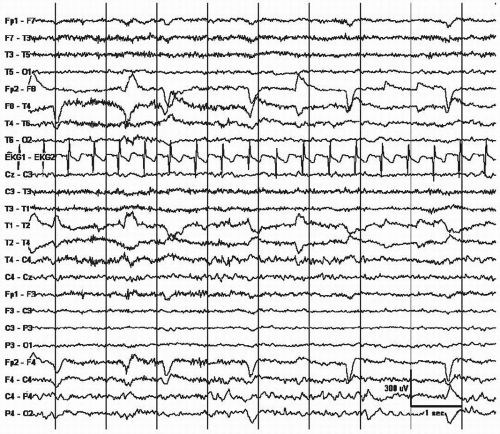 Figure 13.2 Lateral eye movements are noted asymmetrically only from the right due to an enucleated left eyeball (prosthetic eye). |
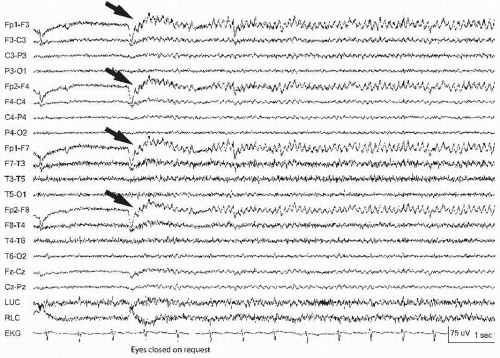 Figure 13.3 Eye blinks can be so rapid that they appear abnormal. Note the bifrontal symmetric 9-Hz activity that represents eye fluttering, elicited with eye closure (arrows). |
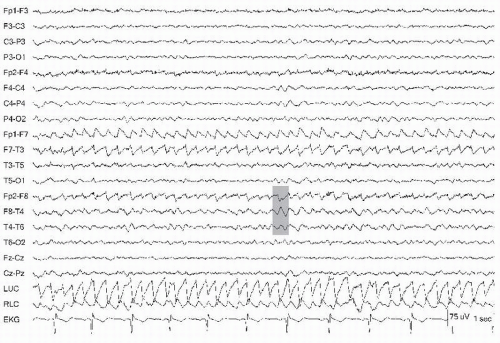
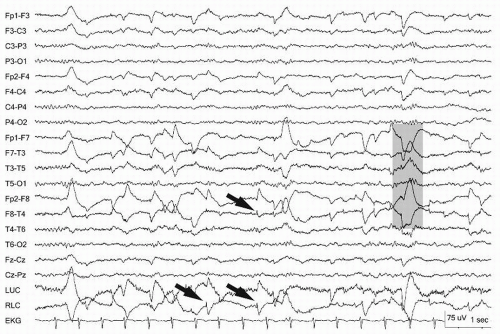 Figure 13.6 Rapid lateral eye movements demonstrated on an awake patient (gray bar). Note the eye leads LUC and RLC, showing the out-of-phase activity. The arrow points to a lateral rectus spike. |
Electroretinogram (ERG)
During photic stimulation, light from the flash stimulus may produce artifact in the frontopolar leads (Fp1/Fp2). This artifact can be mistaken for photic driving due to the entrainment with the stimuli, but is not located occipitally. The source may be the retina (ERG) or from a nonphysiologic source such as a frontopolar electrode with high impedance creating a photocell. Covering the eye with a towel will block the input to the retina (ERG) and this should not be confused with the photoelectric effect (11).
Muscle Artifact
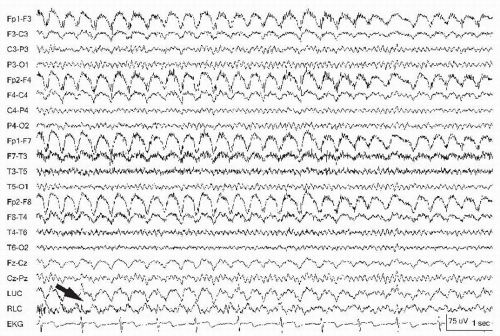
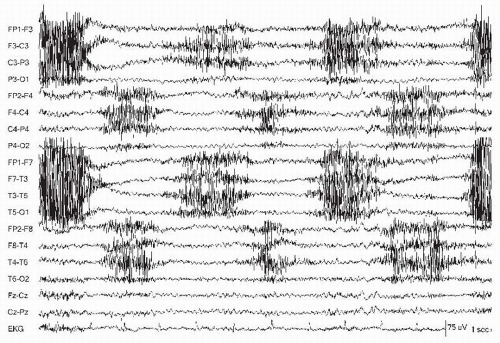
Muscle is another commonly observed artifact in the EEG. It is high-frequency activity noted to be very “spiky” but too fast to be an epileptic discharge. It is observed more while the patient is awake, and may obscure critical portions of the recording, for example, during hypermotor seizures. The frontalis and temporalis muscles are principal sites of myogenic artifact. Frontalis muscle becomes most involved with forced eye closure, and photic stimulation (10). Temporalis muscles become active with jaw clenching, chewing, or bruxism appearing as bursts of fast activity (Fig. 13.9). Technologists can ask the patient to open the mouth that relaxes the jaw and diminishes this artifact. Frontalis muscle contraction during periocular movement may elicit sustained or individual motor unit potentials that can appear like “railroad tracks” (Fig. 13.10). Lateral rectus muscle spikes representing motor unit potentials recorded from the lateral orbit may simulate small interictal discharges (i.e., F7/F8) (Fig. 13.11). During intermittent photic stimulation, eye flutter artifact at frequencies of < 6 Hz may mimic generalized spike-and-wave when coupled with myogenic potentials and create a “pseudo-photoparoxysmal response” (Fig. 13.12) when muscle spikes are time-locked to the flash frequency.
Glossokinetic Artifact
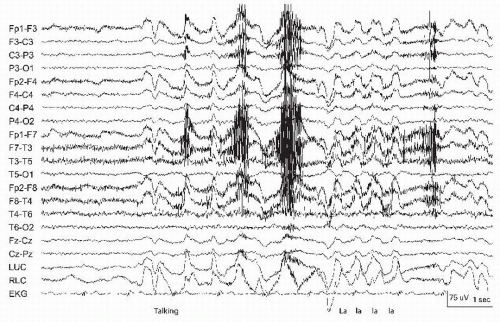
The tongue is a very large muscle centrally located inside the head. Similar to the eye, the tongue creates a bioelectric dipole with the tip of the tongue negative relative to the root (11). During oropharyngeal motion, a direct current (DC) potential is produced that is often diffusely seen with frontal and temporal predominance (11). Cephalad-caudad motions may be produced by tongue movements involuntarily while speaking or when swallowing (Fig. 13.13) producing delta frequency waves that may mimic FIRDA distinguishable by the superimposed muscle artifact. Similar artifact may be produced when patients are asked to say “lilt” or “ta ta ta,” identifying similar patterns as artifact more conclusively (Fig. 13.14). Horizontal tongue movements (i.e., tongue in
cheek) can create unilateral artifact on the EEG that may be confusing. Validation is possible through application of tongue movement monitors with electrodes placed above and below the mouth over the cheek and chin (10). Using a bipolar montage, positive phase reversals are evident with tongue movement in a similar fashion to those recorded during eye movements.
cheek) can create unilateral artifact on the EEG that may be confusing. Validation is possible through application of tongue movement monitors with electrodes placed above and below the mouth over the cheek and chin (10). Using a bipolar montage, positive phase reversals are evident with tongue movement in a similar fashion to those recorded during eye movements.
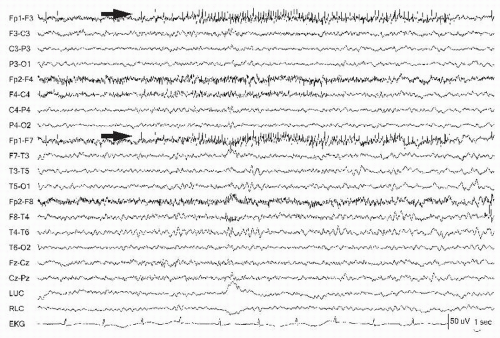 Figure 13.10 The left frontopolar electrode (Fp1) (arrows) demonstrates a motor unit firing that appears like “railroad tracks.” |
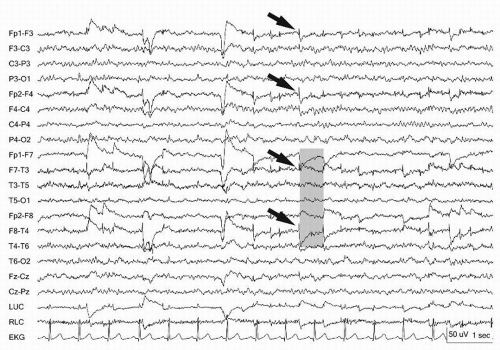 Figure 13.11 Lateral rectus spikes (arrows) are noted bilaterally in the frontopolar leads. Lateral eye movements show out-of-phase deflections at F7 and F8 (gray bar). |
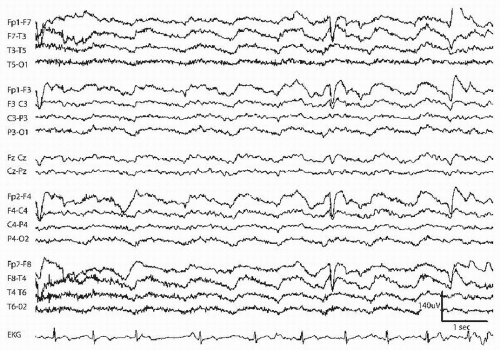 Figure 13.13 Diffuse anterior predominant delta during waking state (eye blinks, muscle) corresponds to drinking (glossokinetic artifact). |
Electrocardiogram (EKG) Artifact
While many artifacts “contaminate” the EEG, some (i.e., the EKG) are essential for interpreting physiologic functions that may occur during the recording session. The heart is another key source of EEG artifact and is variably present on recordings depending on the montage used and the size of the patient. Referential montages often accentuate EKG artifact (Fig. 13.15), especially using ipsilateral ear reference with the larger intra-electrode distances. Linked ears montage can reduce the artifact (Fig. 13.16). Overweight patients with short stocky necks as well as babies may be predisposed to EKG artifact because the dipole is situated closer to the recording electrodes and better able to transmit the current (10). This may be reduced by altering head position. Recording the EKG during routine EEG is essential to enable recognition of the cardiac-cerebral relationship. Channel I of the EKG is approximated with electrodes linking the left and right chest using bipolar recording to identify the QRS. EKG interference may be noted as a small spike at 1/sec that in isolation mimics an interictal spike or in succession and on a low-amplitude record mimics periodic lateralized or generalized periodic epileptiform discharges (PLEDs or GPEDs). Bipolar montages may reveal apparent interictal epileptiform discharges (IEDs) that are “focal” diphasic waveforms predominately in the temporal derivations of the left hemisphere due to the vector created by the electric conduction of the heart. Opposite polarities of the R-wave from the EKG may be seen at the left (negative) and right (positive) ear electrodes. EKG can be readily identifiable as the source because of the regularity and time-locked waves synchronous with the EKG. Importantly, when an electrode is positioned over an artery, the blood pulsing through the vessel moves it regularly to produce a periodic and smooth waveform time-locked to the cardiac rhythm appearing approximately 200 msec after the QRS complex (Fig. 13.17) (10, 11 and 12). Pulse artifact can be eliminated by moving the electrode off the artery.
Sweat
Scalp perspiration will also produce artifact by creating unwanted electric connections between electrodes. Perspiration artifact appears as very low-frequency (<0.5 Hz) low-amplitude undulating waves. Changes in the DC electrode potential from perspiration may result in an unstable baseline (sweat sway) and crossing of tracings in adjacent channels (Fig. 13.18). Perspiration artifacts are usually seen in multiple adjacent channels or over the entire scalp and poses a risk for bridging between electrodes (see also the section “Electrode and Input Lead Artifacts”). They can be reduced somewhat by increasing the low-frequency filter, and by drying the scalp and reapplying electrodes, or by cooling the patient with a fan.
Patient Movements
Patient movements cause the leads or electrodes to move, and thus can provide a large source of “physiologic” artifact on
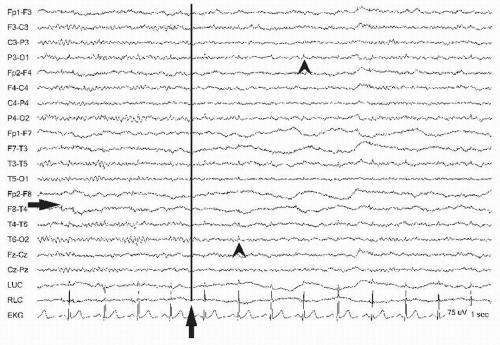
recordings. This is especially true for the awake and ambulatory patient (13), but is also quite notable in those who are agitated and confused. Synchronous video has been inordinately helpful in distinguishing the varieties of patient movements when a technologist is not present for the recording. Regular appearing patient movements such as tremor may manifest at different frequencies and amplitudes such as the 6-Hz tremor of Parkinson disease (Fig. 13.19) or scratching near an electrode. During bipolar recording, extra adjacent electrodes placed on the suspected limb are helpful to confirm tremor artifact but they may be readily apparent in the EKG lead. Sudden or rapid patient movements such as shivering (Fig. 13.20) can mimic the paroxysmal quality of an electrographic seizure, but usually do not show progression. Additional artifact that leads to regular movement of the head or body from large pulses through the aorta or ballistocardiographic artifact gives rise to delayed pulses that can appear more generalized but still linked to the EKG (14). During electrocerebral inactivity recordings at sensitivities of 2 µV/mm, ballistocardiographic artifact and pulse artifact may be more prominent with small scalp arteries causing repetitive movements of electrodes. Stabilizing the head with towels under the neck often eliminates this artifact. Artifact due to respiration is also generated by the movement of the body or the head (Fig. 13.21). This can be seen during hyperventilation over posterior head regions. A technologist can observe the breathing and annotate the recording to indicate where inhalation and exhalation occur (11). In the epilepsy monitoring unit (EMU), the technologist is away from the patient but continuous video and the ability to transmit and display the time-locked behavior with EEG usually leads to accurate interpretation. Technical guidelines for recording in the EMU have been developed by the American Clinical Neurophysiology Society (15). For further discussion, see Chapter 37. Long-term monitoring provides a greater likelihood of deterioration of the patient-electrode interface with drying out of the electrolytic gel or nonadherence of the electrodes to promote artifact that may override interpretation during behavioral events. In the EMU, approximately 20% to 25% of patients with episodes will be manifesting psychogenic nonepileptic attacks (PNEA) (16). These are described in more detail in Chapter 33. The importance of recognizing behavioral sources in addition to historic information is crucial to recognizing nonepileptic events especially if there is “pseudo-evolution” simulating an ictal recording (Fig. 13.22) (4).
recordings. This is especially true for the awake and ambulatory patient (13), but is also quite notable in those who are agitated and confused. Synchronous video has been inordinately helpful in distinguishing the varieties of patient movements when a technologist is not present for the recording. Regular appearing patient movements such as tremor may manifest at different frequencies and amplitudes such as the 6-Hz tremor of Parkinson disease (Fig. 13.19) or scratching near an electrode. During bipolar recording, extra adjacent electrodes placed on the suspected limb are helpful to confirm tremor artifact but they may be readily apparent in the EKG lead. Sudden or rapid patient movements such as shivering (Fig. 13.20) can mimic the paroxysmal quality of an electrographic seizure, but usually do not show progression. Additional artifact that leads to regular movement of the head or body from large pulses through the aorta or ballistocardiographic artifact gives rise to delayed pulses that can appear more generalized but still linked to the EKG (14). During electrocerebral inactivity recordings at sensitivities of 2 µV/mm, ballistocardiographic artifact and pulse artifact may be more prominent with small scalp arteries causing repetitive movements of electrodes. Stabilizing the head with towels under the neck often eliminates this artifact. Artifact due to respiration is also generated by the movement of the body or the head (Fig. 13.21). This can be seen during hyperventilation over posterior head regions. A technologist can observe the breathing and annotate the recording to indicate where inhalation and exhalation occur (11). In the epilepsy monitoring unit (EMU), the technologist is away from the patient but continuous video and the ability to transmit and display the time-locked behavior with EEG usually leads to accurate interpretation. Technical guidelines for recording in the EMU have been developed by the American Clinical Neurophysiology Society (15). For further discussion, see Chapter 37. Long-term monitoring provides a greater likelihood of deterioration of the patient-electrode interface with drying out of the electrolytic gel or nonadherence of the electrodes to promote artifact that may override interpretation during behavioral events. In the EMU, approximately 20% to 25% of patients with episodes will be manifesting psychogenic nonepileptic attacks (PNEA) (16). These are described in more detail in Chapter 33. The importance of recognizing behavioral sources in addition to historic information is crucial to recognizing nonepileptic events especially if there is “pseudo-evolution” simulating an ictal recording (Fig. 13.22) (4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree