Chapter 9 Atypical parkinsonism, parkinsonism-plus syndromes, and secondary parkinsonian disorders
Introduction
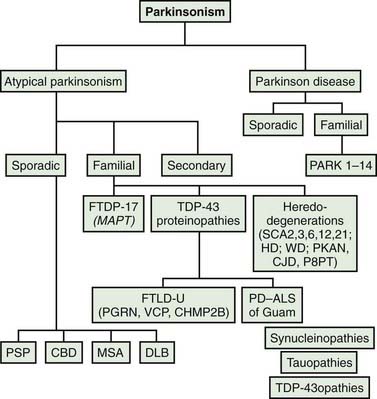
Most patients who are referred to specialized movement disorder clinics with hypokinetic disorders are diagnosed clinically as having Parkinson disease (PD) (Table 9.1) (Jankovic et al., 2000). The second most common group of parkinsonian patients is categorized clinically as having parkinsonism-plus disorders and pathologically as having multiple system degenerations (Fahn, 1977; Jankovic, 1989; Stacy and Jankovic, 1992; Jankovic, 1995b; Jankovic et al., 2000; Litvan et al., 2003; Abdo et al., 2006) (Fig. 9.1). There is, however, a growing body of evidence to support the emerging classification of neurodegenerative disorders according to pathogenetic mechanisms into (1) amyloidoses (e.g., Alzheimer disease, or AD), (2) ubiquitin-proteasome disorders (e.g., PD, parkin PD), (3) synucleinopathies (e.g., PD, multiple system atrophy, or MSA), (4) tauopathies (e.g., frontotemporal dementia (FTD) with parkinsonism, or FTDP; progressive supranuclear palsy, or PSP; corticobasal degeneration, or CBD), (5) polyglutamine expansion diseases (e.g., Huntington disease, spinocerebellar atrophies (SCAs)), and (6) prion diseases (e.g., Creutzfeldt–Jakob disease) (Jankovic, 2008). As our understanding of the mechanisms of these diseases advances, refinements in this classification and new categories of disease will undoubtedly emerge. Besides parkinsonian findings, patients with these disorders exhibit additional (“plus”) features. For example, supranuclear ophthalmoparesis typifies patients with PSP; dysautonomia and ataxia are typically present in MSA; laryngeal stridor occurs in striatonigral degeneration (SND); a combination of apraxia, cortical myoclonus, and “alien hand” occurs in CBD; dementia-parkinsonism occurs not only in PD dementia (PDD), but also in dementia with Lewy bodies (DLB) and AD; and dementia coupled with motor neuron disease occurs in parkinsonism–dementia–amyotrophic lateral sclerosis complex of Guam (Table 9.1). As there are no biologic markers for any of these disorders, the diagnostic criteria are based on the presence of certain clinical features and neuropathologic confirmation (Cummings, 2003; Litvan et al., 2003). While levodopa continues to be the most effective drug for the treatment of motor symptoms associated with PD, a minority (about a third) of patients with atypical parkinsonism also respond to levodopa, although “responsiveness” has not been well defined in the literature (Constantinescu et al., 2007).
Table 9.1 Classification of parkinsonism
Rarely, psychogenic causes have been implicated in the pathogenesis of parkinsonism (Lang et al., 1995). This chapter focuses only on the sporadic (nongenetic) forms of multisystem degenerations. The secondary and heredodegenerative causes of parkinsonism are covered elsewhere in this book and in other reviews.
Progressive supranuclear palsy
Clinical features and natural history
First described by Steele, Richardson, and Olszewski (Steele et al., 1964; Steele, 1972; Williams et al., 2008) in 1964, progressive supranuclear palsy (PSP) has become a well-characterized, distinct clinical-pathologic entity (Jankovic et al., 1990a; Golbe, 1993; Collins et al., 1995; Litvan, 1998b; Golbe et al., 2007; Golbe and Ohman-Strickland, 2007; Houghton and Litvan, 2007; Azher and Jankovic, 2008). The first volume solely devoted to PSP was published in 1993 (Litvan and Agid, 1993), and a summary of the 1999 “First Brainstorming Conference on PSP” was published (Litvan et al., 2000). The diagnosis of PSP should be considered in any patient with progressive parkinsonism and disturbance of ocular motility (Jankovic, 1984a; Maher and Lees, 1986; Jankovic et al., 1990a; Friedman et al., 1992; Cardoso and Jankovic, 1994).
PSP is considered to be a sporadic disorder, but familial PSP has been reported (Brown et al., 1993; de Yebenes et al., 1995; Tetrud et al., 1996; Rojo et al., 1999; Kaat et al., 2009). The supranuclear ophthalmoparesis was not well documented in the familial cases, and the presence of atypical features, such as early cognitive decline in the family reported by Brown and colleagues (1993) and the relatively early age at onset (53 years) in the de Yebenes and colleagues (1995) kindred, suggested that these families had a neurodegenerative disorder that was distinct from idiopathic PSP. Furthermore, no linkage to the tau gene has been identified in any of these families, although linkage to 1q31.1 has been demonstrated in one large Spanish family (Ros et al., 2005). In a comprehensive epidemiologic-genetic study, 33% of individuals with PSP were found to have at least one first-degree relative with dementia or parkinsonism and 12 families with PSP were identified (5 cases were pathologically confirmed), but the intrafamily phenotype was very variable, the P301L MAPT mutation was identified in only one patient, and there were other limitations in obtaining the family and medical information (Kaat et al., 2009). While it is possible that homozygous mutations occur, such as deletion at codon 296 in MAPT gene identified in one atypical case of PSP (Ferrer et al., 2003), the presence of subhaplotypes overrepresented in individuals with PSP increases the risk of the disease.
In a review of 126 PSP patients, unsteadiness of gait, frequent falling, monotonous speech, loss of eye contact, slowness of movement and of mentation, sloppy eating habits, and nonspecific visual difficulty were the most typical presenting features (Jankovic et al., 1990a). The earliest and most disabling symptom of PSP usually relates to gait and balance impairment, as a result of which patients frequently fall and sustain injuries. The average period from onset of symptoms to the first fall in PSP is 16.8 months, as compared to 108 months in PD, 42 months in MSA, 54 months in dementia with Lewy bodies, and 40.8 months in vascular parkinsonism (Williams et al., 2006). The marked instability is presumably a result of visual-vestibular impairment, axial rigidity, and bradykinesia (Jankovic et al., 1990a). Using computerized posturography, we demonstrated that measures of balance impairment can reliably differentiate between PSP and PD even in early stages of the disease (Ondo et al., 2000). In contrast to the short and shuffling steps, stooped posture, narrow base, and flexed knees that are typically seen in PD, PSP patients have a stiff and broad-based gait, with a tendency to have their knees (and trunk) extended and arms slightly abducted. Instead of turning en bloc, they tend to pivot, which further compromises their balance (Video 9.1). Some PSP patients may present with the syndrome of pure akinesia, also referred to by some as motor blocks (Matsuo et al., 1991; Giladi et al., 1992; Riley et al., 1994) and gait ignition failure (Atchison et al., 1993; Nutt et al., 1993), which is manifested chiefly by akinesia of gait (start hesitation, freezing, motor blocks, festination, disequilibrium with frequent falling), marked impairment of speech (stuttering, stammering, hypophonia), handwriting difficulty (micrographia), and eyelid motor disturbance (blepharospasm, eyelid freezing) without rigidity, tremor, or dementia and without response to levodopa. Primary progressive freezing of gait or pure akinesia with gait freezing may be the initial, main, or the only presentation of PSP (Compta et al., 2007; Williams et al., 2007b; Facheris et al., 2008; Williams and Lees, 2009). Some of the patients with pure freezing may also have PD, pallidonigroluysian degeneration (Ahmed et al., 2008), CBD, and diffuse Lewy body disease (DLBD) (Factor et al., 2006; Williams et al., 2007b). In addition to PSP, frontal gait disorder may be the initial manifestation of AD and CBD (Rossor et al., 1999). Although the PSP may be associated with ataxia and the gait may appear ataxic, the patients usually do not exhibit prominent cerebellar findings. The uncompensated loss of postural reflexes and motor blocks (freezing) especially on turning, coupled with a peculiar lack of insight into the difficulties with equilibrium (possibly secondary to frontal lobe dysfunction), leads to frequent falling. There is, however, also emerging evidence of cerebellar abnormalities, both clinically and pathologically, in PSP (Kanazawa et al., 2009). Abnormal otolith-mediated reflexes may also contribute to the falls of PSP (Liao et al., 2008). Loss of insight, particularly inability to accurately predict performance on future tasks (anticipatory awareness), is a common feature not only in PSP but also in patients with FTD and CBD (O’Keeffe et al., 2007). This is often present even in the early stages of the disease and helps to differentiate PSP from PD (Litvan et al., 1996c). Although the motor subscale of the Unified Parkinson’s Disease Rating Scale (UPDRS) has been found to reliably assess most aspects of PSP (Cubo et al., 2000), the PSP Rating Scale (PSPRS) has been found to be sensitive to disease progression, increasing at a mean rate of about 1 point per month (Golbe and Ohman-Strickland, 2007). PSPRS, along with the Quality of Life Scale (Schrag et al., 2006b), should be used in future clinical trials investigating novel therapeutic interventions. ![]()
Along with postural instability, supranuclear ophthalmoparesis typically manifested by paralysis of downgaze is the most important distinguishing sign of PSP (Litvan et al., 1997c) (Video 9.2). The predictability of these two features with regard to the final pathologic diagnosis was confirmed by a clinical-pathologic study of 24 autopsy-proven cases of PSP (Litvan et al., 1996b). About one-third of PSP patients complain of blurred vision, diplopia, and eye discomfort, but most eventually lose their ability to read or maintain eye contact (Friedman et al., 1992). Involuntary persistence of ocular fixation is a typical, though rarely mentioned, feature of PSP. Other oculomotor abnormalities that are seen in patients with PSP include impairment of saccades, optokinetic nystagmus, and the presence of square wave jerks (Rascol et al., 1991). In early stages of PSP, patients might have only mild limitation of voluntary downgaze and inability to converge, but slowing of horizontal and vertical saccades (also demonstrated by optokinetic nystagmus) appears to be the earliest oculomotor sign of PSP (Video 9.3). Indeed, slowing of vertical saccades, even in the presence of normal vertical saccade amplitude, was found to be an early sign of an autopsy-proven PSP (Hardwick et al., 2009). One study compared saccades, optokinetic nystagmus, and other ophthalmologic signs in six patients with PSP compared to PD and normal control (Garbutt et al., 2004). All PSP patients showed slowing of vertical saccade and quick phase of nystagmus; square wave jerks were more frequent and larger during fixation; vertical optokinetic nystagmus showed impaired slow wave response, and quick phases were slowed and combined with square wave jerks. Deficient generation of the motor command by midbrain burst neurons has been suggested as the primary mechanism for the slow vertical saccades (Bhidayasiri et al., 2001). Slowing of vertical saccades might help to differentiate PSP from other parkinsonian disorders, including PD, MSA, and CBD, although some slowing of vertical saccades can be seen occasionally also in these parkinsonian disorders (Vidailhet et al., 1994; Rivaud-Péchoux et al., 2000; Bhidayasiri et al., 2001). In addition to slow vertical saccades, bilateral impairment of the antisaccade task (the patient is instructed to look in the direction opposite to the visual stimulus) correlates well with frontal lobe dysfunction in PSP (Vidailhet et al., 1994) and other neurodegenerative and frontal lobe disorders (Condy et al., 2004; Munoz and Everling, 2004; Zee and Lasker, 2004). Abnormalities in antisaccades imply a dysfunction of the dorsolateral prefrontal cortex and the superior colliculus (Condy et al., 2004). Later, limitation of vertical and then lateral eye movements follows. The ophthalmoparesis can be overcome by the oculocephalic (doll’s eye) maneuver (Videos 9.1, 9.2, and 9.4), but with disease progression and brainstem involvement, vestibulo-ocular reflexes can be lost, suggesting additional nuclear involvement (Ishino et al., 1974). ![]()
Several clinical-pathologic studies have attempted to establish criteria that separate PSP from other, related disorders. In 60 cases of patients clinically diagnosed with PSP, 47 (78%) of which were pathologically proven, false-positive diagnoses included PD combined with cortical Lewy body pathology or AD, MSA, CBD, Pick disease, motor neuron disease, cerebrovascular disease, and FTD (Osaki et al., 2004). The application of NINDS-SPSP diagnostic criteria (Litvan et al., 1996a) and other criteria improved the accuracy of initial clinical diagnosis only marginally. On the basis of an analysis of 103 pathologically confirmed consecutive cases of PSP, Williams and colleagues (2005) divided PSP into two categories: Richardson syndrome, characterized by the typical features described in the original report, and PSP-P, in which the clinical features overlap with PD and the course is more benign. The latter group, representing about a quarter of all patients with PSP (Williams et al., 2005), has less tau pathology than the classic Richardson syndrome (Williams et al., 2007a, 2008; Williams and Lees, 2009). The mean 4R-tau/3R-tau ratio of the isoform composition of insoluble tangle-tau isolated from the pons was significantly higher in Richardson’s syndrome (2.84) than in PSP-P syndrome (1.63). Further studies are needed to confirm or refute this classification. Another subgroup of PSP that has been identified is the so-called “frontal” PSP, representing about 20% of all PSP patients (Kaat et al., 2007). These patients initially present with behavioral and cognitive symptoms, with or without ophthalmoparesis, and then they evolve into typical PSP.
Pathologically documented cases of PSP without ophthalmoparesis have been reported (Davis et al., 1985; Collins et al., 1995; Daniel et al., 1995). When PSP patients with ophthalmoparesis were compared with those without ophthalmoparesis, no differences in the pathology of the two groups were noted, and there was no correlation between the severity of clinical symptoms and degenerative changes (Daniel et al., 1995). In another pathologic study, brains of patients with PSP who had gaze palsy had two-fold greater loss of neurons in the substantia nigra pars reticulata (SNr) (Halliday et al., 2000). Since SNr projects to the superior colliculi, degeneration of SNr might contribute to the limitation of eye movements. Supranuclear ophthalmoparesis may occur also in DLB (Lewis and Gawel, 1990; Fearnley et al., 1991; De Bruin et al., 1992; Daniel et al., 1995; Brett et al., 2002), CBD (Gibb et al., 1990), postencephalitic parkinsonism, prion disease, Wernicke encephalopathy, dorsal midbrain syndrome, paraneoplastic syndrome, progressive subcortical gliosis, Whipple disease (Jankovic, 1986; Simpson et al., 1995; Averbuch-Heller et al., 1999), Niemann–Pick and Gaucher disease (Shulman et al., 1995; Uc et al., 2000), Kufor-Rakeb syndrome (PARK9; secondary to mutation in ATP13A2 gene on chromosome 1p36) (Hampshire et al., 2001; Schneider et al., 2010), stiff-person syndrome (Oskarsson et al., 2008), primary pallidal degeneration, and other disorders (Calabrese and Hadfield, 1991).
Pseudobulbar symptoms in PSP patients are characterized chiefly by dysarthria, dysphagia, and “emotional incontinence” (Video 9.5). Rigidity, bradykinesia, and hypertonicity of the facial muscles produce deep facial folds and a typical worried or astonished facial expression (Jankovic, 1984b) (Fig. 9.2). The worried appearance is partly due to contraction of the procerus (and possibly corrugator) muscle, the so-called “procerus signs” (Romano and Colosimo, 2001) (Videos 9.1 and 9.2). Speech in PSP is characterized by a spastic, hypernasal, hypokinetic, ataxic, monotonous, low-pitched dysarthria (Kluin et al., 1993) (Videos 9.1, 9.4, and 9.5). The speech rate may be slow or fast, and some patients have severe palilalia and stuttering. An “apraxia of phonation” has been reported in one patient who was aphonic except during periods of excitement or during sleep (Jankovic, 1984b). In contrast, some patients have almost continuous involuntary vocalizations, including loud groaning, moaning, humming, and grunting sounds (Jankovic et al., 1990a). Progressive dysphagia causes most patients to modify their diet, and some eventually need a feeding gastrostomy to maintain adequate nutrition. As a result of chewing difficulties, inability to look down, and poor hand coordination, PSP patients are often described as “sloppy eaters.” ![]()
In a review of dystonia in pathologically proven cases of PD, MSA, and PSP, Rivest and colleagues (1990) found dystonia to be an uncommon feature, noted in only 15 of 118 (13%) cases. They regarded the frequently reported neck extension as a form of axial rigidity rather than dystonia. In another study, the increased neck muscle tone was thought to have features of both dystonia (tonic shortening reaction) and rigidity (antagonist muscle contraction indicative of increased tonic stretch reflex) (Tanigawa et al., 1998). Neck extension, although often noted in published reports, is actually an uncommon sign in PSP. Indeed, neck flexion, usually associated with MSA (Quinn, 1994), can occasionally be seen in PSP (Daniel et al., 1995). In contrast to the typical presence of neck rigidity, truncal muscle tone is only slightly increased, and distal limbs may actually seem hypotonic (Tanigawa et al., 1998). In some patients, however, distal dystonia can be seen (Barclay and Lang, 1997). Although PSP is usually a symmetrical disorder, dystonia represents an occasional exception in that unilateral dystonia may be present, particularly in the more advanced stages of the disease. The most common form of dystonia in PSP is blepharospasm. In one study, 29% of patients had involuntary orbicularis oculi contractions producing blepharospasm, and over one-third had “apraxia” of eyelid opening, eyelid closure, or both (Friedman et al., 1992). Although some (Lepore and Duvoisin, 1985) have hypothesized that these lid abnormalities are due to involuntary supranuclear inhibition of levator palpebrae, others have drawn attention to the similarity of this disorder of eyelid motor control to the parkinsonian phenomenon of sudden transient freezing, hence suggesting the term lid freezing (Jankovic, 1995a). Other terms that are used to describe this condition include pretarsal blepharospasm (Elston, 1992) and focal eyelid dystonia (Krack and Marion, 1994). In one study of 83 patients with PSP, 38 (46%) had some form of dystonia, 22 (24%) had blepharospasm, 22 (27%) had limb dystonia, and 14 (17%) had axial dystonia (Barclay and Lang, 1997). Sometimes, spontaneous arm levitation, a well-recognized sign in CBD, is also seen in patients with PSP and may be wrongly attributed to dystonia (Barclay et al., 1999).
In their original monograph, Steele, Richardson, and Olszewski (1964) indicated that mild dementia was present during early stages of the disease. Though some investigators have reported severe cognitive impairment in this population (Pillon and Dubois, 1993), others have attributed these deficits, at least in part, to poor visual processing (Fisk et al., 1982; Rafal et al., 1988; Jankovic et al., 1990a; Daniel et al., 1995). Despite a relative preservation of short-term memory, cognitive slowing, impairment of executive (goal-directed) functions, and subcortical dementia with deficits in tasks requiring sequential movements, conceptual shifts, and rapid retrieval of verbal knowledge are typically present in patients with PSP (Johnson et al., 1991; Pillon and Dubois, 1993; Litvan et al., 1998e). The memory disturbance that is found in patients with PSP is similar to that of patients with PD and Huntington disease, but it is markedly different from that of patients with AD (Pillon et al., 1994). The apathy, with or without depression, and other hypoactive behaviors that are typically seen in PSP have been attributed to a dysfunction in the frontal cortex and associated circuitry (Litvan et al., 1998e). This is in contrast to Huntington disease, in which behaviors such as agitation, anxiety, and irritability have been related to hyperactivity of the medial and orbitofrontal cortical circuitry. Sparing of olfactory function in PSP, in contrast to that in PD, is another clinical difference between the two neurodegenerative disorders (Doty et al., 1993). Litvan and colleagues (1996d) studied the neuropsychiatric aspects of PSP in 22 patients and found that apathy occurred in 91%, disinhibition in 36%, dysphoria in 18%, anxiety in 18%, and irritability in fewer than 9%. Another sign of frontal lobe dysfunction in PSP is the “applause sign” (signe de l’applaudissement), which probably represents a perseveration of automatic behavior (Dubois et al., 1995, 2005) (Video 9.6). This sign, characteristically present in patients with PSP (but also present in some patients with FTD with parkinsonism and CBD), is manifested by persistence (perseveration) of clapping after the patient is instructed to clap consecutively three times as quickly as possible. In a study of patients with various neurologic disorders evaluated at Baylor College of Medicine, the applause sign was present in 77.8% of 9 patients with CBD, 53.9% of 13 patients with MSA, 52.6% of 19 patients with PSP, 20% of 10 patients with Huntington disease, and 12.5% of 24 patients with PD (Wu et al., 2008). Although the test differentiated patients with CBD from those with PD (P < 0.005) and HD (P < 0.005), it failed to discriminate patients with PSP from other parkinsonian groups, but had a 100% specificity in distinguishing parkinsonian patients from normal subjects. ![]()
After idiopathic PSP, the most common cause of PSP is a multi-infarct state. Multi-infarct or vascular PSP can be difficult to differentiate clinically from the more common idiopathic variety (Dubinsky and Jankovic, 1987; Stern et al., 1989; Winikates and Jankovic, 1994; Rektor et al., 2006). In addition to a much higher frequency of stroke risk factors and abnormal imaging studies, the vascular PSP patients are more likely to have asymmetric and predominantly lower-body involvement, cortical and pseudobulbar signs, dementia, and bowel and bladder incontinence (Winikates and Jankovic, 1994). The concept of vascular PSP is supported by the observation by Ghika and Bogousslavsky (1997), who found that 81% of patients with clinically diagnosed PSP had hypertension. A clinical-pathologic study of four patients who were clinically diagnosed with PSP but found to have vascular PSP at autopsy showed that vascular PSP is characterized by asymmetric signs, falls within 1 year of onset, and vascular lesions on magnetic resonance imaging (MRI) (Josephs et al., 2002). In addition, three of the four patients carried the H2 tau haplotype, whereas 93.7% of patients with idiopathic PSP carry the H1 tau haplotype (78.4% of controls carry this haplotype) (see later). Reported causes of secondary PSP include exposure to organic solvents (McCrank et al., 1989), paraneoplastic syndrome (Jankovic, 1985), mesencephalic tumor (Siderowf et al., 1998), surgery on aorta (Mokri et al., 2004), and other rare and often unsubstantiated causes.
The relentlessly progressive course leads to death, usually from aspiration, within 10 years of onset in the majority of cases. In one clinical-pathologic study of 24 patients with PSP, the median survival from onset was 5.6 years, and this was shorter in men and in patients who experienced falls during the first year of symptoms and with early dysphagia or supranuclear palsy (Litvan et al., 1996c; Litvan, 2003). Overall, the median latency from onset to chairbound state is 5 years and to death is 7 years (Golbe and Ohman-Strickland, 2007).
In another clinical-pathologic study involving 16 cases of PSP, Birdi and colleagues (2002) found the mean survival to be 8.6 years (range: 3–24) years, and the mean age at death was 72.3 years (range: 60–89). The early onset, presence of falls, slowness, and early downward gaze palsy correlated with a rapid progression (Santacruz et al., 1998). In a review of 187 cases, those with early bulbar features had around 5 years less life expectancy than those who had no or late bulbar features (Nath et al., 2003). Similar to other series, the median survival in this study was 5.7 years. Since this figure is based on deceased cases, it might be too pessimistic because slowly progressive cases are still being followed. In another study of 50 PSP patients, Goetz and colleagues (2003) found the median survival from the onset of first symptom to be 7.9 years (6.5 years in the 21 patients who were followed to death). In addition to the short survival, PSP is associated with many symptoms that have a serious impact on the quality of life (Schrag et al., 2006b).
Epidemiology
About 6% of all parkinsonian patients who are evaluated in a specialized clinic fulfill the clinical criteria for PSP. On the basis of a medical record review of the Rochester Epidemiology Project, the average annual incidence rate has been estimated to be 5.3 new cases per 100 000 person-years (Bower et al., 1997). The prevalence, after age adjustment to the US population, has been estimated to be 1.39 per 100 000 (Golbe et al., 1988). In a review of computerized records of 15 general practices in and around London, Schrag and colleagues (1999) found an age-adjusted prevalence for PSP of 6.4 per 100 000. In other studies carried out in the United Kingdom, the prevalence of PSP ranged from 1 to 6.5 per 100 000 (Nath et al., 2001). Similar to the European studies, a prevalence of 5.82 per 100 000 has been reported in Yonago, Japan (Kawashima et al., 2004).
Like PD, PSP occurs more often in men, but its onset at a mean age of 63 years is about 10 years later than the typical onset of PD. Although no well-designed epidemiologic studies have been performed in PSP, one case-control study found that PSP patients were more likely to live in areas of relatively sparse population (Davis et al., 1988). Another study by the same investigators failed to identify any risk factor, except for low likelihood of completing at least 12 years of education, that would differentiate patients with PSP from a matched control population (Golbe et al., 1996).
Neurodiagnostic studies
Electrophysiologic studies have been helpful in documenting other abnormalities, such as sleep difficulties and seizures. Polysomnographic evaluation of 10 patients with moderate to severe PSP revealed marked sleep abnormalities; all had significant periods (2–6 hours) of insomnia (Aldrich et al., 1989). Sleep problems were correlated with worsening dementia. Another study showed marked reduction in percentage of REM sleep (Montplaisir et al., 1997). The same study also showed frontal electroencephalogram (EEG) slowing in patients with PSP. In a review of 62 patients seen over a 9-year period, Nygaard and colleagues (1989) noted seizures in 7 patients and suggested a higher-than-expected frequency of seizures in this population. This has not been our observation, but the relatively high frequency of seizures reported by Nygaard and colleagues (1989) might have been secondary to cortical infarcts. Abnormalities in motor and frontal sensory evoked potentials have been found in 8 of 13 patients with the clinical diagnosis of PSP (Abbruzzese et al., 1991).
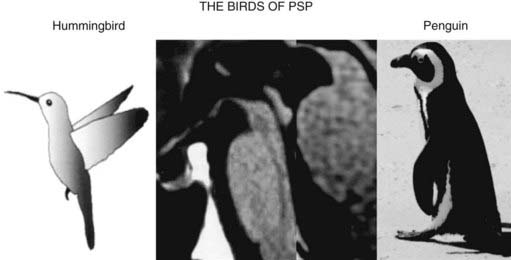
The typical findings on computed tomography or MRI scans of patients with PSP include generalized and brainstem, particularly midbrain, atrophy (Stern et al., 1989; Soliveri et al., 1999). Measuring the anteroposterior diameter of the suprapontine midbrain, Warmuth-Metz and colleagues (2001) found that in contrast to PD patients (mean 18.5 mm), PSP patients had a significantly lower diameter (13.4 mm) on axial T2-weighted MRI, and as a result, the authors concluded that this finding reliably differentiates between PD and PSP and recommended that this evaluation “should be incorporated into the diagnostic criteria for PSP.” In another study, utilizing midsagittal MRI, the average midbrain area of patients with PSP was 56 mm2, which was significantly smaller than that of patients with PD (103 mm2) or MSA-P (97.2 mm2), and this parameter, particularly the ratio of the area of the midbrain to the area of the pons, was found to reliably differentiate among the three disorders (Oba et al., 2005). On the midsagittal view of the MRI, as a result of atrophy of the rostral midbrain tegmentum, the most rostral midbrain, the midbrain tegmentum, the pontine base, and the cerebellum appear to correspond to the bill, head, body, and wing, respectively, of a hummingbird (Kato et al., 2003) or penguin (Oba et al., 2005; Sitburana and Ondo, 2009) (Fig. 9.3). The “hummingbird sign” was demonstrated in all 8 MRI scans of PSP patients but not in any of the 12 scans of PD patients or 10 scans of normal controls (Kato et al., 2003). The “morning glory sign,” a peculiar MRI finding of midbrain atrophy with concavity of the lateral margin of the midbrain tegmentum, resembling the lateral margin of the morning glory flower, is observed in PSP patients with supranuclear gaze palsy (Adachi et al., 2004). Using diffusion-weighted MRI (DWI-MRI), Seppi and colleagues (2003) were able to differentiate between PSP and PD with 90% sensitivity and 100% specificity, but this technique could not differentiate between PSP and MSA. Using diffusion tensor imaging and voxel-based morphometry in PSP, Padovani et al. (2006) provided evidence of both gray and white matter degeneration even in early stages of PSP. Using voxel-based morphometry in 15 patients with clinically proven PSP and 14 with CBD, distinct patterns of atrophy were observed that differentiated between the two disorders with 93% accuracy (Boxer et al., 2006). CBD patients had marked asymmetric (L > R) pattern of atrophy involving premotor cortex, superior parietal lobules, and striatum, whereas PSP was characterized by atrophy of the midbrain, pons, thalamus, and striatum.
Stroke risk factors and a multi-infarct state on computed tomography or MRI have been noted in patients with PSP with a higher frequency than in those with PD (Dubinsky and Jankovic, 1987). One etiology for a subgroup of PSP might be small vessel disease producing subcortical ischemia with reduction of regional cerebral blood flow, cerebral hypometabolism, and a multi-infarct state. MRIs in patients with PSP, MSA, and other parkinsonian syndromes have been associated with putaminal hypointensity on T2-weighted MRI, but this finding is less consistently noted in PSP than in the other parkinsonism-plus syndromes (Drayer et al., 1989; Stern et al., 1989). In one study, PSP could be differentiated from MSA by the presence of marked atrophy and hyperintensity of the midbrain as well as atrophy of the frontal and temporal lobes (Schrag et al., 2000).The “eye of the tiger” sign on brain MRI, typically associated with neurodegeneration with brain iron accumulation type 1 (NBIA1), formerly Hallervorden–Spatz disease (see later), has been also reported in PSP (Davie et al., 1997). PSP is associated with dorsal midbrain atrophy, and as a result of degeneration of superior colliculi, the floor of the third ventricle is flattened on sagittal MRI images (Savoiardo et al., 1994).
Positron emission tomography (PET) scanning has revealed decreased metabolic activity in the caudate, putamen, and prefrontal cortex (Foster et al., 1988; Goffinet et al., 1989; Blin et al., 1990), but the earliest sign of PSP appears to be decreased glucose metabolism in the midbrain (Mishina et al., 2004). Uptake of 18F-dopa is usually reduced in PSP but may be normal in early stages (Bhatt et al., 1991). This suggests that the parkinsonian findings in early PSP are related more to postsynaptic receptor changes than to a loss of presynaptic dopamine terminals. In another study, 18F-dopa uptake was markedly reduced in the caudate as well as in the anterior and posterior putamen of PSP patients (Brooks et al., 1990a). In contrast, the uptake was reduced only in the posterior putamen in PD patients. Similarly, dopamine transporter, imaged by [11C]-WIN PET, showed a relatively uniform reduction involving the entire striatum, whereas patients with PD had involvement chiefly of the posterior putamen (Ilgin et al., 1999). F-dopa and F-deoxyglucose PET were abnormal in 5 (33%) individuals among 15 subjects at risk of familial PSP even though they did not (yet) exhibit any symptoms (Piccini et al., 2001). Using 11C-raclopride as a D2 ligand, Brooks and colleagues (1992) showed a 24% reduction in D2 density in the caudate and a 9% reduction in the putamen of patients with PSP. A discriminant analysis of striatal 18F-dopa PET studies indicates that this technique can reliably differentiate between PD and PSP, but it is less accurate in differentiating between PD and MSA (Burn et al., 1994). By using [11C]diprenorphine, significantly reduced opioidergic binding has been demonstrated in both caudate and putamen, whereas the binding is essentially normal in PD and reduced in the putamen but not the caudate in SND (Burn et al., 1995). The cortical muscarinic acetylcholine receptors, as measured by PET and [11C]N-methyl-4-piperidyl benzilate, were found to be normal in a series of patients with PSP (Asahina et al., 1998). Using [123I]β-CIT SPECT, Pirker and colleagues (2000) showed marked reduction of striatal binding in PD, PSP, MSA, and CBD, but the pattern of abnormality (reduction in overall binding and asymmetry) did not allow a differentiation between the various disorders. Using the receptor ligand [18F]altanserin, PET scans in 8 patients with PSP showed upregulation of 5-HT2A receptors in the substantia nigra and to a lesser degree in the striatum, as compared to 13 controls, and these changes significantly correlated with the UPDRS-III and PSP-Rating Scale (Stamelou et al., 2009).
In addition to imaging studies, analysis of the CSF may also be helpful in evaluation of patients with PSP. One study, for example, found a low ratio of light (33 kDa) to heavy (55 kDa) tau protein in the CSF of patients with PSP, which differentiated (without any overlap) this group from other tauopathies such as AD, CBD, and FTD and from synucleinopathies such as PD and DLB (Borroni et al., 2008). This finding had an 87% sensitivity and 86% specificity and was validated against MRI voxel-based morphometry of brainstem gray matter.
Neuropathology, neurochemistry, and pathogenesis
The motor, neurobehavioral, and neuro-ophthalmic findings seen in patients with PSP reflect marked neuronal degeneration in the basal nucleus of Meynert, pallidum, subthalamic nucleus, superior colliculi, mesencephalic tegmentum, substantia nigra pars compacta (SNc) and SNr, locus coeruleus, red nucleus, reticular formation, vestibular nuclei, cerebellum, and spinal cord (Steele et al., 1964; Steele, 1972; Zweig et al., 1987; Juncos et al., 1991; Jellinger and Bancher, 1993; Hardman et al., 1997a, 1997b; Kanazawa et al., 2009). In addition, in contrast to PD, both the globus pallidus interna (GPi) and externa (GPe) are markedly affected in PSP, and this may contribute to thalamic inhibition and some of the parkinsonian features (Hardman and Halliday, 1999a, 1999b). Along with the degeneration of the SNc and the pedunculopontine tegmental nucleus, the glutamatergic caudal intralaminar thalamic nuclei are involved in both PSP and PD (Henderson et al., 2000). In addition there is a widespread loss of A10 dopamine neurons, clearly more severe than in PD (Murphy et al., 2008). Atrophy of the superior cerebellar peduncle has been found to be a frequent finding in the brains of patients with PSP, and this correlates with the duration of the disease (Tsuboi et al., 2003b). In one pathologic study, marked atrophy of the GPi differentiated PSP from PD and DLB (Cordato et al., 2000). Cerebellar degeneration and tau-positive inclusion bodies have been demonstrated in the Purkinje cells of patients with PSP, particularly when associated clinically with ataxia (Kanazawa et al., 2009). Although bladder dysfunction has not been considered a prominent feature in patients with PSP, the finding of neuronal loss in Onuf’s nucleus in patients with PSP suggests that bladder function should be carefully evaluated in patients with PSP (Scaravilli et al., 2000).
On the basis of a workshop sponsored by the National Institutes of Health, the following neuropathologic criteria were proposed: (1) high-density neurofibrillary tangles (NFTs) and neuropil threads in the basal ganglia and brainstem and (2) tau-positive astrocytes or their processes in areas of involvement (Hauw et al., 1994; Litvan et al., 1996a). Using rather crude pathologic criteria, De Bruin and Lees (1994) demonstrated that the clinical manifestations of PSP can vary considerably. They reviewed 90 cases reported in the literature between 1951 and 1992 that met the following two criteria: subcortical neurofibrillary degeneration and exclusion of other recognized nosologic entities. There were 51 men and 34 women (in 5 cases, the gender was not specified), with an average age at onset of 62 years and mean age at death of 67 years. The most common symptoms were unsteady gait (70.7%), stiffness (67.4%), slurred speech (67.4%), falls (60.6%), dysphagia (57.3%), and blurring of vision (21%). Vertical gaze palsy was the most common sign but was noted in only 68.5% of all cases, followed by bradykinesia in 67.4%, dysarthria in 67.4%, rigidity in 58.4%, axial dystonia in 48.3%, segmental dystonia in 20.2%, and tremor in 16.8%.
Microscopic examination reveals NFTs, granulovacuolar degeneration, gliosis, and rare Lewy bodies (Steele et al., 1964; Steele, 1972; De Bruin and Lees, 1994). One pathologic study showed no evidence of increased Lewy bodies in PSP (Tsuboi et al., 2001). The NFTs in PSP differ from those seen in AD and other neurodegenerative disorders in that PSP tangles consist of 15 nm straight tubules rather than 20–24 nm wide paired helical filaments (PHF) (Joachim et al., 1987; Jellinger and Bancher, 1993; Dickson, 1997). PHFs are composed of the microtubule-associated protein tau (MAPT) in a hyperphosphorylated state (Goedert et al., 1996). In contrast to the other neurodegenerative diseases with tau pathology, such as AD, Pick disease, CBD, and the parkinsonism–dementia complex of Guam, which are characterized by flame-shaped NFTs, the NFTs in PSP are predominantly of the globose type. The tau-containing astrocytic inclusions (“tufted astrocytes”) are more common in the basal ganglia and the brainstem of PSP, whereas they are more common in the cortex of brains of CBD. Tau inclusions in PSP oligodendrocytes are described as “coiled bodies.” Tsuboi and colleagues (2003a) showed that APOE ε4 is a risk factor for Alzheimer-type pathology in PSP. Some brains of patients with Guam parkinsonism–dementia complex also contain deposits of the TDP-43 protein (TAR-DNA binding protein 43), which binds ubiquitin, suggesting that a common pathogenic mechanism through conformational changes in this protein links this disease with FTDP, ALS, and related neurodegenerative diseases (Hasegawa et al., 2007).
The clinical and pathologic overlap between PSP, CBD, and AD provides evidence that these disorders are closely related, although the absence of amyloid deposits in PSP and CBD suggests a clear difference between the two disorders and AD (Feany and Dickson, 1996; Litvan et al., 1999; Rossor et al., 1999; Boeve et al., 2003a; Wakabayashi and Takahashi, 2004; Galpern and Lang, 2006). Among 180 cases of clinically diagnosed PSP that came to autopsy, only 137 (36%) met the pathologic criteria for PSP (Josephs and Dickson, 2003). The other diagnoses included CBD, MSA, DLBD, and Creutzfeldt–Jakob disease (CJD). The following features were seen more frequently in non-PSP cases than in PSP cases: tremor, psychosis, early dementia, asymmetric findings, absence of H1 haplotype, and presence APOE ε4. The various disorders can be differentiated by a careful histologic examination. For example, the tau in NFTs of AD shows marked ubiquitin immunoreactivity, whereas the NFT-tau from PSP does not (Flament et al., 1991; Shin et al., 1991). While the abnormal tau in AD consists chiefly of 55, 64, and 68 kDa forms, the PSP tau consists only of 64 and 68 kDa forms (Conrad et al., 1997) (Fig. 9.4). Cdk5, a kinase that is physiologically involved in the phosphorylation of tau protein, is overexpressed in PSP brains (Borghi et al., 2002). PSP and CBD also overlap with FTD in clinical, pathologic, biochemical, and genetic aspects (Boeve et al., 2003a). Tuft-shaped astrocytes seem to be more indicative of PSP than CBD, with prominent tuft-shaped astrocytes in the precentral gyrus and premotor cortex, caudate, putamen and globus pallidus, red nucleus, and superior colliculus in PSP brains (Hattori et al., 2003). Another pathologic evidence of overlap between PSP and CBD is the abundance of pathologic tau in the white matter in both disorders (Zhukareva et al., 2006). The histopathologic features of PSP also overlap closely with those of postencephalitic parkinsonism (Litvan et al., 1998c) and the parkinsonism–dementia complex of Guam, but the pallidum and the subthalamic nucleus are usually spared in the former, and the cortex seems to be more involved in the latter. Rarely, cases with clinical presentation nearly identical to that of PSP have been reported to have the pathologic picture of pallidonigroluysial atrophy (PNLA) (Kosaka et al., 1981; Ahmed et al., 2008). Of 400 cases of PSP, 8 (2%) were found to have pathologic features of PSP and PNLA is now considered a rare variant of PSP, with the latter usually presenting at a younger age, with gait and handwriting abnormalities in early stages, and having slower progression (Ahmed et al., 2008). In one study, 54% of pathologically proven cases of PSP had coexistent AD and PD, providing evidence for overlap between these neurodegenerative disorders (Gearing et al., 1994). Calbindin-D28k immunoreactivity, normally found in the medium-sized neurons and neuropil of the striatal matrix, GP, and SNr, is reduced in the GP of patients with PSP (and in striatum and SNr of patients with SND) (Ito et al., 1992). This finding suggests that calcium cytotoxicity might play a role in the marked neuronal degeneration that is found in these structures.
PSP and CBD also share pathologic tau doublet (64 and 69 kDa) as well as the predominance of 4R tau isoforms with argyrophilic grain disease (AGD). This sporadic neurodegenerative disease, which accounts for about 5% of all cases of dementia, is manifested by late-onset episodic memory loss and progressive dementia along with personality changes, irritability, agitation, delusions, and apathy. Neither clinical nor neuroimaging features can reliably differentiate between AGD and AD, and the diagnosis of AGD is based on autopsy findings. Pathologically, aggregated tau proteins are found in limbic structures in the shape of distinct argyrophilic grains and coiled bodies (Tolnay et al., 2002; Liang et al., 2005; Ferrer et al., 2008). Because of overlap in pathologic features with not only PSP and CBD, but also AD and other neurodegenerative diseases, questions have been raised whether AGD represents a distinct entity. Argyrophilic grains are present in 4–9% of adult brains and the incidence increases with age. Besides aging, oxidative stress appears to play a critical role in the development of the pathologic changes, including activation of tau kinases, which facilitate tau hyperphosphorylation in some neurons. The abnormal tau apparently binds to p62 and, after ubiquitation, aggregates, forming grains and tangles. Thrombin, which accumulates in the grains, has been thought to facilitate tau truncation, which further increases oxidative stress and contributes to the toxicity.
Molecular misreading of the ubiquitin-B (UBB) gene results in a dinucleotide deletion in ubiquitin-B mRNA, which in turn leads to accumulation of the mutant protein ubiquitin-B+1 in AD, Pick disease, FTD, PSP, and AGD, but not in synucleinopathies (PD or MSA) (Fischer et al., 2003) (Fig. 9.1). This finding provides evidence that the ubiquitin-proteasome system is impaired in these tauopathies and that ubiquitin-B+1 protein serves as a marker for these diseases. Unique haplotype in 17q21, found in 16% of Spanish as well as American PSP patients but not in any of the controls, provides further evidence that PSP is a form of a tauopathy (Pastor et al., 2004). Better understanding of the molecular pathways that are altered in various neurodegenerative disorders could be helpful in differential diagnosis based on postmortem examination of brain tissue. Using microarray technology in substantia nigra (SN) samples from six patients with PD, two patients with PSP, one patient with FTDP, and five controls, Hauser and colleagues (2005) found 142 genes that were differentially expressed in PD cases and controls, 96 in the combination of PSP-FTDP, and 12 genes that were common to all three disorders.
The marked reduction in striatal D2 receptors in PSP, demonstrated by PET studies, has also been documented in postmortem studies (Ruberg et al., 1993). In contrast to the D2 receptors, the striatal D1 receptors are spared (Pierot et al., 1988; Pascual et al., 1992). Biochemical studies show that in addition to degeneration of the nigrostriatal dopaminergic system, the cholinergic and GABAergic systems seem to be particularly affected. Cholinergic neurons have been found to degenerate in the Edinger–Westphal nucleus, the rostral interstitial nucleus of Cajal (possibly contributing to the extensor nuchal rigidity), the medial longitudinal fasciculus (contributing to vertical gaze palsy), the superior colliculus, and the pedunculopontine nucleus (Zweig et al., 1987; Juncos et al., 1991). Autoradiographic study of 18 brains of patients with pathologically confirmed PSP showed marked reduction in M2 and M4 receptors in the posterior striatal cholinergic interneurons and a reduction of M4 receptors on medium spiny projection neurons, confirming marked cholinergic dysfunction in PSP compared to Lewy body dementia (n = 45), Alzheimer disease (n = 39), and normal controls (n = 50) (Warren et al., 2007). Using the technique of an in-situ hybridization of GAD67 messenger RNA, Levy and colleagues (1995) demonstrated 50–60% reduction in the number of neurons expressing GAD67 messenger RNA in the caudate nucleus, ventral striatum, and both segments of the GP in three brains of patients with PSP. They suggest that the marked destruction of the basal ganglia output nuclei might explain the poor response to dopaminergic therapy in this disorder.
The most striking neurochemical abnormality found in PSP brains is a marked reduction in striatal dopamine, dopamine receptor density, choline acetyltransferase activity, and loss of nicotinic, rather than muscarinic, cholinergic receptors in the basal forebrain (Young, 1985; Pierot et al., 1988; Ruberg et al., 1993). Normal dopamine levels in the nucleus accumbens suggest that the mesolimbic system is relatively spared. Because of the relative sparing of the mesocorticolimbic dopaminergic system in contrast to the severe degeneration of the mesostriatal system, some investigators have suggested that the primary site of pathology in PSP is the striatum and that the changes observed in the SN are simply a result of a retrograde degeneration (Ruberg et al., 1993). The cholinergic neurons are, however, also markedly affected and may be primarily involved in PSP. The cholinergic innervation of the thalamus is particularly affected in PSP, and this finding helps to differentiate PSP from PD (Shinotoh et al., 1999). Also, reduction in acetylcholine vesicular transporter has been found to differentiate PSP from other types of neurodegenerative disorders (Suzuki et al., 2002). Suzuki and colleagues (2002) were able to correlate reductions in acetylcholine vesicular transporter and choline acetyltransferase activity in the striatum of postmortem brains of patients with PSP, but choline acetyltransferase was also significantly reduced in the inferior frontal cortex. Glutamate has been found to be increased in the striatum, pallidum, nucleus accumbens, and occipital and temporal cortex. In contrast to PD, glutathione was found to be increased in the SN of PSP patients (Perry et al., 1988). The observation that multiple neurotransmitters, particularly dopamine and acetylcholine, are affected in PSP suggests that PSP is not a primary neurotransmitter disease but a disorder in which multiple subpopulations of neurons degenerate for yet unknown reasons.
The cause of PSP is unknown, but oxidative damage, mitochondrial dysfunction, and abnormal protein (e.g., tau) processing have received the most attention (Albers and Augood, 2001). Decreased rates of adenosine triphosphate production were found in a preliminary study of muscle mitochondria function in patients with PSP, suggesting impaired mitochondrial respiratory chain activity in PSP (Di Monte et al., 1994). Further support for mitochondrial defect in PSP was later provided by additional studies. Using cybrid lines expressing mitochondrial genes, Swerdlow and colleagues (2000) found a 12.4% decrease in complex I activity (P < 0.005) in cybrid (cytoplasmic hybrid) cells but no change in complex IV activity. Cybrid cells also had significantly increased levels of several antioxidant enzymes. This study suggests a mtDNA-encoded electron transport chain enzyme defect in PSP. Further evidence for a defect in mitochondrial oxidative metabolism is the finding of significantly reduced levels of phosphocreatine and Mg2+ and increased levels of adenosine diphosphate and inorganic phosphate using phosphorus magnetic resonance spectroscopy of the brain and calf muscle in five PSP patients (Martinelli et al., 2000). Using proton magnetic resonance spectroscopic imaging, Tedeschi and colleagues (1997) found a reduced N-acetylaspartate/creatine-phosphocreatine ratio in the brainstem, centrum semiovale, and frontal and precentral cortex and N-acetylaspartate/choline in the lentiform nucleus in patients with PSP.
Research into mechanisms and treatment of PSP has been hampered by paucity of suitable animal models. Rats that are exposed systemically and chronically to annonacin, a lipophilic mitochondrial complex I inhibitor extracted from tropical fruit plants, have been shown to produce neurodegeneration resembling PSP, providing further evidence of mitochondrial dysfunction in PSP (Champy et al., 2004). Another potential animal model of PSP is a transgenic mouse (TgT34) which overexpresses wild-type human tau (T34 tau isoform) driven by the astrocyte-specific glial fibrillary acidic protein promoter (Forman et al., 2005). These transgenic mice accumulate tau in astrocytes, similar to what occurs in PSP, leading to focal neuronal degeneration. Another potential animal model of PSP is the JNPL3 mouse in which the human four repeat tau with the P301L mutation is expressed resulting in a severe phenotype within 5–6 months but without motor defects (Lewis et al., 2000).
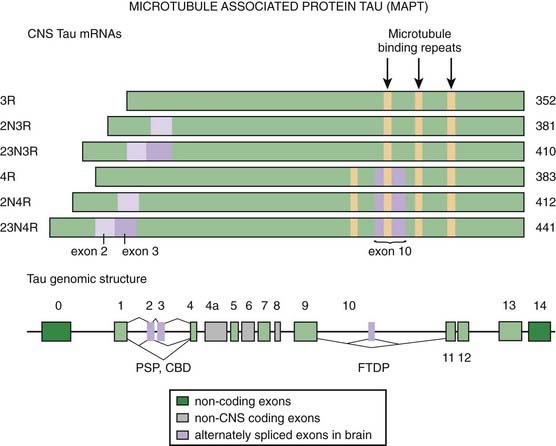
Recent studies have drawn attention to abnormal phosphorylation of tau proteins as an important mechanism of neurodegeneration in PSP. Tau exon 10 + 16 mutation in the tau gene (MAPT, IVS10, C-U, +16) was found in a case of young-onset (age 40 years) of PSP phenotype with neuropathologic features of FTD (Morris et al., 2003). Tau is phosphorylated by serine, threonine, and tyrosine kinases, and this phosphorylation might lead to abnormal aggregation. There is growing support for the notion of altered regulation of tau gene expression in PSP (Rademakers et al., 2005). The relationship between abnormalities in tau and PSP is described in detail in the section on tauopathies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree