Figure 65-1 Ganglionic AChR autoantibody level and percentage of anhidrosis. A, A dramatic improvement of the percentage of anhidrosis was observed during intravenous immunoglobulin treatment. B, Subsequent plasma exchange courses resulted in a decrease of antibody level to values at or near the normal range.
Because of worsening autonomic function and persistent symptoms, the patient underwent two courses of IVIg, following which her autonomic function tests improved and anhidrosis was markedly improved (TST% fell to 3% anhidrosis) (Fig. 65-2). Autoantibody was only mildly improved and remained elevated (0.29 nmol/L). During the course of the plasma exchange followed by mycophenolate for 3 months at 1000 mg twice daily, her clinical deficits progressively improved and the antibody concentrations decreased to values within the normal range (0.05, 0.07, 0 nmol/L).
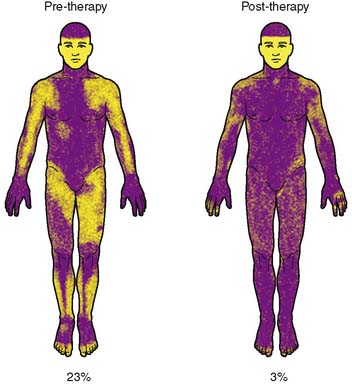
Figure 65-2 Thermoregulatory sweat test at onset (left) and following treatment with intravenous immunoglobulin (right). The percentage below the figures indicates the percentage of anhidrosis on the thermoregulatory sweat test.
CONCLUSION
This case illustrates a number of key issues of AAG. She has the typical phenotype of AAG associated with elevated ganglionic AChR antibody titers. These patients typically have autonomic failure with prominent parasympathetic failure. Typically these patients have widespread anhidrosis, heat intolerance, Adie’s pupils, and bowel and bladder failure as manifestations of AAG.
Autonomic function tests are also typical with widespread anhidrosis and increased levels of ganglionic AChR antibody. Response to immunotherapy supports an autoimmune etiology. There remains, however, major uncertainties on therapy. There are no treatment trials to confirm efficacy of any form of immunotherapy. Some cases (such as this one) seem to respond to IVIg and others apparently do not, and seem instead to respond to plasma exchange. It is the unproven impression of the authors that some patients seem to require a prolonged duration of treatment of 3 to 12 months with immunotherapy, but this also is based on personal experience and not scientific evidence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








