Chapter 10 Brain Stem
The brain stem consists of the medulla oblongata, pons and midbrain. It is sited in the posterior cranial fossa, and its ventral surface lies on the clivus. It contains numerous intrinsic neurone cell bodies and their processes, some of which are the brain stem homologues of spinal neuronal groups. These include the sites of termination and cells of origin of axons that enter or leave the brain stem through the cranial nerves. They provide the sensory, motor and autonomic innervation of structures that are mostly in the head and neck. Autonomic fibres, which arise from the brain stem, are distributed more widely. Additional groups of neurones receive input related to the special senses of hearing, vestibular function and taste (Ch. 12). The reticular formation is an extensive and often ill-defined network of neurones that extends throughout the length of the brain stem and is continuous caudally with its spinal counterpart. Some of its nuclei are concerned with cardiac, respiratory and alimentary control; some are involved in aspects of many neural activities, and others provide or receive massive afferent and efferent cerebellar projections.
The brain stem is the site of termination of numerous ascending and descending fibres and is traversed by many others. The spinothalamic (spinal lemniscal), medial lemniscal and trigeminal systems ascend through the brain stem to reach the thalamus (see Figs 8.32, 10.22). Prominent corticospinal projections descend through the brain stem, and corticobulbar projections end within it (see Fig. 8.41).
Overview of Cranial Nerves and Cranial Nerve Nuclei
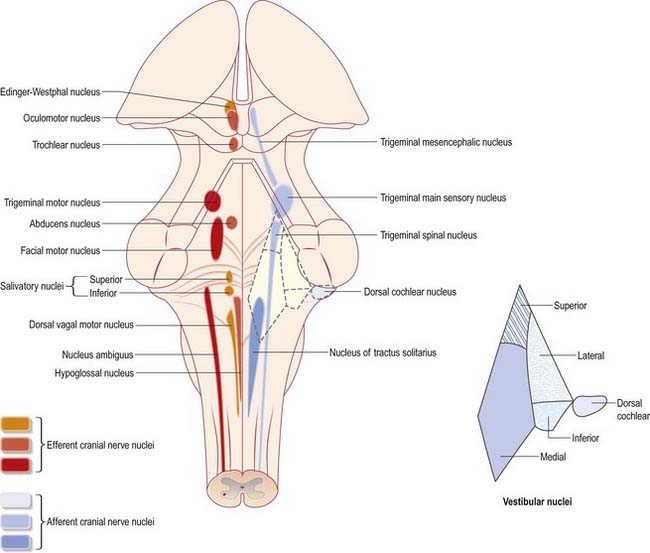
The cranial nerves are individually named and numbered (using roman numerals) in a rostrocaudal sequence (see Table 1.1). Cranial nerve I (olfactory) terminates directly in cortical and subcortical areas of the frontal and temporal lobes. It is closely associated functionally with the limbic system and is described in that context (Ch. 16). The fibres of cranial nerve II (optic) pass into the optic chiasma and emerge as the optic tract, which terminates in the lateral geniculate nucleus of the thalamus. Cranial nerves III (oculomotor) and IV (trochlear) attach to the midbrain. Cranial nerve V (trigeminal) attaches to the pons, medial to the middle cerebellar peduncle. Cranial nerves VI (abducens), VII (facial) and VIII (vestibulocochlear) attach to the brain stem at or close to the junction of the pons and the medulla. Cranial nerves IX (glossopharyngeal) and X (vagus), the cranial part of cranial nerve XI (accessory) and cranial nerve XII (hypoglossal) all attach to the medulla.
Cranial nerves III to XII, which attach to the brain stem, are associated with a number of cell groupings of varying size, referred to collectively as the cranial nerve nuclei (Fig. 10.1). The nuclei are either the origin of efferent cranial nerve fibres or the site of termination of cranial nerve afferents. For convenience, they are considered to be organized into six discontinuous, longitudinal cell columns that correspond to the columns that can be identified in the embryo (see Fig. 1.4). Three columns are ‘sensory’ and three are ‘motor’ in function.
Medulla Oblongata
External Features and Relations
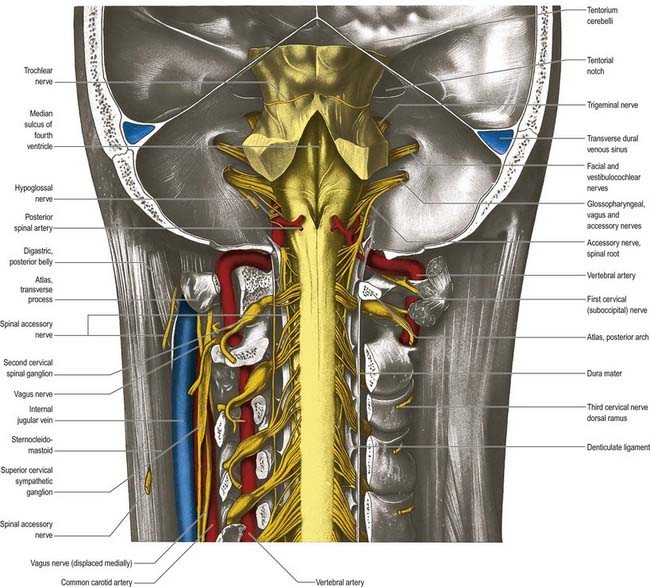
The medulla oblongata extends from the lower pontine margin to a transverse plane that is above the first pair of cervical spinal nerves and intersects the upper border of the atlas dorsally and the centre of the dens ventrally (Fig. 10.2). It is approximately 3 cm long and 2 cm in diameter at its widest. The ventral surface of the medulla is separated from the basilar part of the occipital bone and apex of the dens by the meninges and occipito-axial ligaments. Caudally, the dorsal surface of the medulla occupies the midline notch between the cerebellar hemispheres.
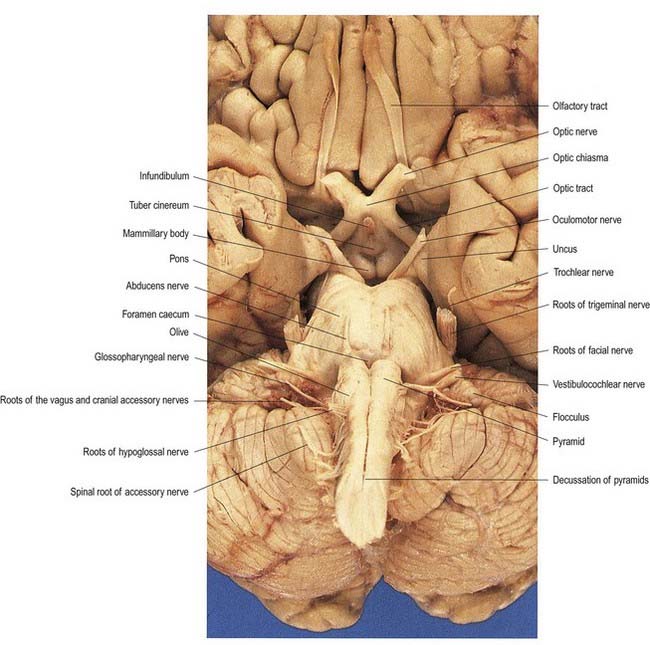
The ventral and dorsal surfaces of the medulla (Fig. 10.3; see also Fig. 5.11) possess a longitudinal median fissure and sulcus, respectively, which are continuous with their spinal counterparts. Caudally, the ventral median fissure is interrupted by the obliquely crossing fascicles of the pyramidal decussation. Rostrally, it ends at the pontine border in a diminutive depression, the foramen caecum. Immediately lateral to the ventral median fissure is a prominent elongated ridge called the pyramid, which contains descending pyramidal, or corticospinal, axons (see Fig. 10.3). The lateral margin of the pyramid is indicated by a shallow ventrolateral sulcus. From this emerges, in line with the ventral spinal nerve roots, a linear series of rootlets that constitute the hypoglossal nerve. The abducens nerve emerges at the slightly narrowed rostral end of the pyramid, where it adjoins the pons. Caudally, the pyramid tapers into the spinal ventral funiculus. Lateral to the pyramid and the ventrolateral sulcus is an oval prominence, the olive (Figs 10.3, 10.4), which contains the inferior olivary nucleus. Lateral to the olive is the posterolateral sulcus. The glossopharyngeal, vagus and accessory nerves join the brain stem along the line of this sulcus, in line with the dorsal spinal nerve roots.
The spinal central canal extends into the caudal half of the medulla, migrating progressively more dorsally until it opens out into the lumen of the fourth ventricle. This divides the medulla into a closed part, which contains the central canal, and an open part, which contains the caudal half of the fourth ventricle (see Figs 10.2, 5.11).
In the closed part of the medulla, a shallow posterointermediate sulcus on either side of the dorsal median sulcus, continuous with its cervical spinal counterpart, indicates the location of the ascending dorsal columns (fasciculus gracilis and fasciculus cuneatus). The ascending fasciculi are at first parallel to each other, but at the caudal end of the fourth ventricle they diverge, and each develops an elongated swelling, the gracile and cuneate tubercles, produced by the subjacent nuclei gracilis and cuneatus, respectively (Figs 10.5, 10.6). Most fibres in the fasciculi synapse with neurones in their respective nuclei, and these project to the contralateral thalamus, which in turn projects to the primary somaesthetic cortex (see Fig. 8.32). The inferior cerebellar peduncle forms a rounded ridge between the caudal part of the fourth ventricle and the glossopharyngeal and vagal rootlets. The two peduncles diverge and incline to enter the cerebellar hemispheres, where they are crossed by the striae medullares, which run to the median ventricular sulcus (see Fig. 5.11). Here also the peduncles form the anterior and rostral boundaries of the lateral recess of the fourth ventricle. This becomes continuous with the subarachnoid space through the lateral apertures of the fourth ventricle, the foramina of Luschka. A tuft of choroid plexus, continuous with that of the fourth ventricle, protrudes from the foramina on either side. The fibre composition of the inferior cerebellar peduncle is described in Chapter 13.
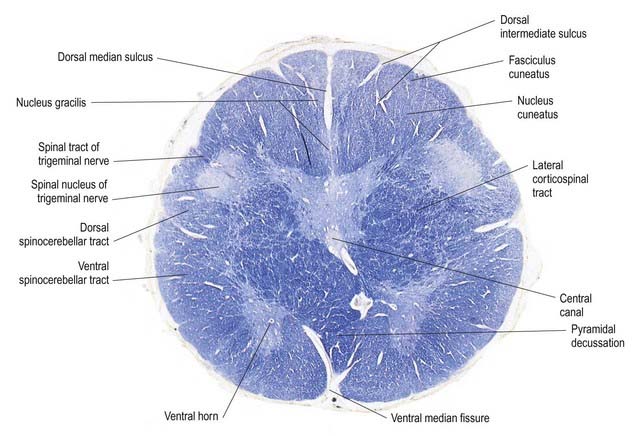
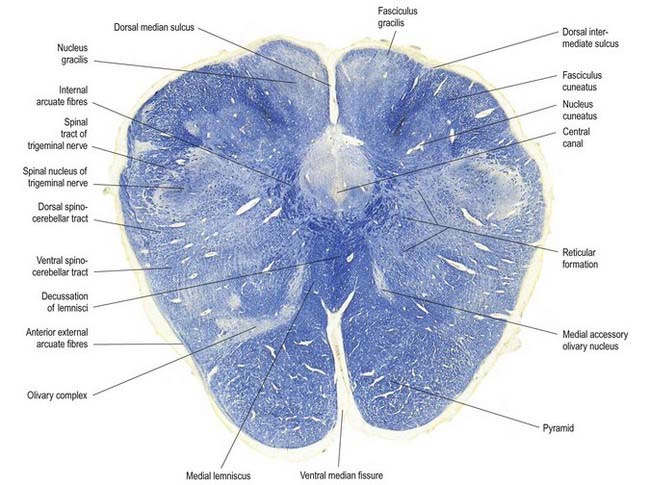
Fig. 10.5 Transverse section through the medulla oblongata at the level of the pyramidal decussation.
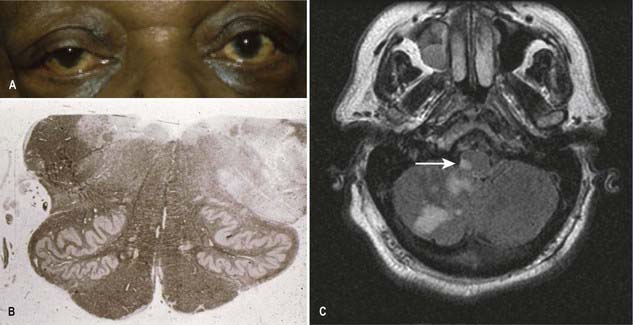
CASE 1 Downbeat Nystagmus and Arnold–Chiari Malformation
A 24-year-old woman presents with a long history of increasing headache, blurred vision when attempting to read and an increasingly unsteady gait with intermittent falls. Neurological examination reveals downbeat nystagmus with the eyes in the primary position, amplified by down-gaze; dysmetria of the lower extremities with heel-to-shin testing; and hyperreflexia in both lower extremities.
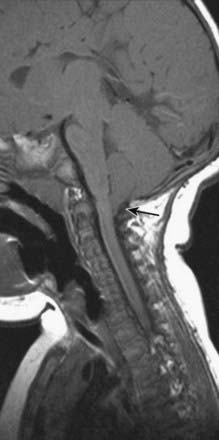
Magnetic resonance imaging (MRI) shows ‘beaking’ of the dorsal midbrain and enlargement of the lateral and third ventricles, with herniation of the cerebellar tonsils through the foramen magnum. See Figure 10.7.
A ventriculoperitoneal shunt is placed, with marked symptomatic improvement.
Internal Structure
Transverse Section of the Medulla at the Level of the Pyramidal Decussation
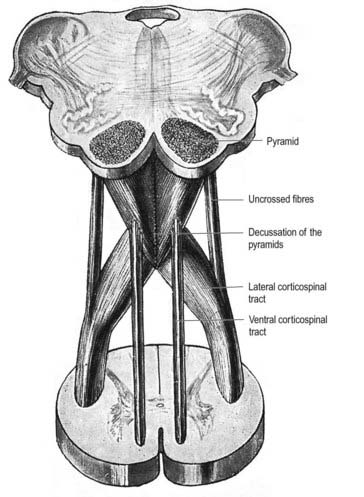
A transverse section across the lower medulla oblongata (see Fig. 10.5) intersects the dorsal, lateral and ventral funiculi, which are continuous with their counterparts in the spinal cord. The ventral funiculi are separated from the central grey matter by corticospinal fibres, which cross in the pyramidal decussation to reach the contralateral lateral funiculi (see Fig. 10.11). The decussation displaces the ventral intersegmental tract, the central grey matter and the central canal dorsally. Continuity between the ventral grey column and central grey matter, which is maintained throughout the spinal cord, is lost. The column subdivides into the supraspinal nucleus (continuous above with that of the hypoglossal nerve), which is the efferent source of the first cervical nerve, and the spinal nucleus of the accessory nerve, which provides some spinal accessory fibres and merges rostrally with the nucleus ambiguus.
Transverse Section of the Medulla at the Level of the Decussation of the Medial Lemniscus
The medullary white matter is rearranged above the level of the pyramidal decussation (see Fig. 10.6). The pyramids contain ipsilateral corticospinal and corticonuclear fibres, the latter distributed to nuclei of cranial nerves and other medullary nuclei. At this level, they form two large ventral bundles flanking the ventral median fissure. The accessory olivary nuclei and lemniscal decussation are dorsal.
The medial lemniscus ascends from the lemniscal decussation on each side as a flattened tract near the median raphe. As the tracts ascend, they increase in size because fibres join from upper levels of the decussation. Corticospinal fibres are ventral, and the medial longitudinal fasciculus and tectospinal tract are dorsal. Fibres are rearranged in the decussation, so that those from the nucleus gracilis come to lie ventral to those from the nucleus cuneatus. Above this, the medial lemniscus is also rearranged, with ventral (gracile) fibres becoming lateral and dorsal (cuneate) fibres medial. At this level, medial lemniscal fibres show a laminar somatotopy on a segmental basis, in that fibres from C1 to S4 spinal segments are segregated sequentially from medial to lateral.
The nucleus of the spinal tract of the trigeminal nerve (see Fig. 10.22) is separated from the central grey matter by internal arcuate fibres; it is separated from the lateral medullary surface by the trigeminal spinal tract, which ends in it, and by some dorsal spinocerebellar tract fibres. The latter progressively incline dorsally and enter the inferior cerebellar peduncle at a higher level.
Two other nuclei occur at this level. One is dorsolateral to the pyramid, and the other is medial to it and near the median plane. These are parts of the precerebellar medial accessory olivary nucleus, described with the inferior olivary nuclear complex. Precerebellar nuclei of the vestibular, pontine and reticular system are described in Chapter 13.
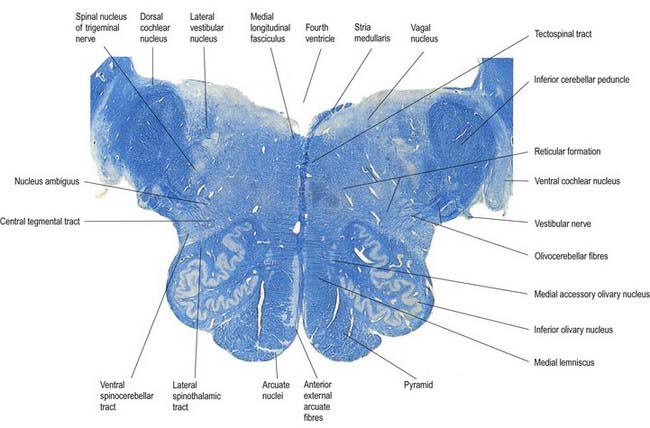
Transverse Section of the Medulla at the Caudal End of the Fourth Ventricle
A transverse section level with the lower end of the fourth ventricle shows some new features, along with most of those already described (Fig. 10.8). The total area of grey matter is increased by the presence of the large olivary nuclear complex and nuclei of the vestibulocochlear, glossopharyngeal, vagus and accessory nerves.
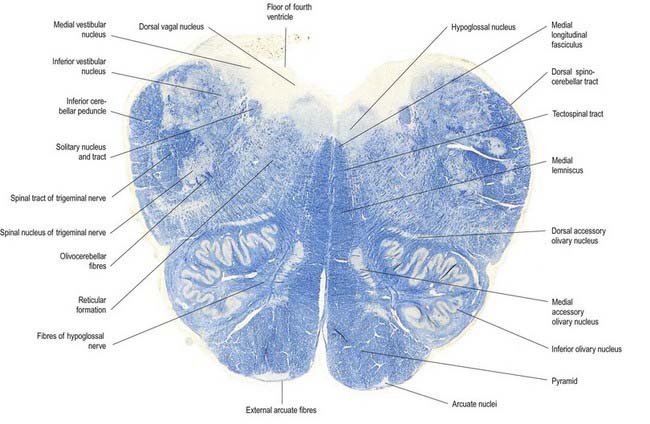
Fig. 10.8 Transverse section through the medulla oblongata at the caudal end of the fourth ventricle.
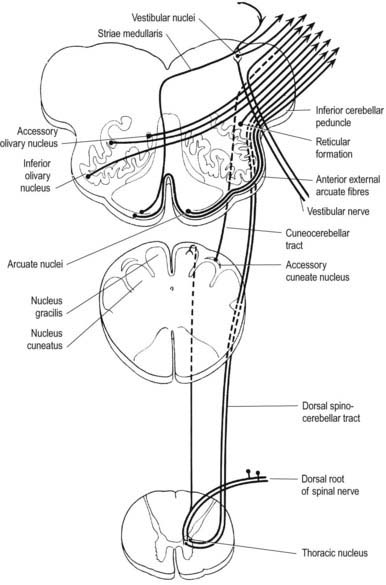
A smooth, oval elevation—the olive—lies between the ventrolateral and dorsolateral sulci of the medulla. It is formed by the underlying inferior olivary complex of nuclei and lies lateral to the pyramid, separated from it by the ventrolateral sulcus and emerging hypoglossal nerve fibres. The roots of the facial nerve emerge between its rostral end and the lower pontine border, in the cerebellopontine angle. The arcuate nuclei are curved, interrupted bands, ventral to the pyramids, and are said to be displaced pontine nuclei. Anterior external arcuate fibres and those of the striae medullares are derived from them. They project mainly to the contralateral cerebellum through the inferior cerebellar peduncle (Fig. 10.9).
The tractus solitarius and its associated circumferential nucleus solitarius extend throughout the length of the medulla. The tract is composed of general visceral afferents from the vagus and glossopharyngeal nerves. The nucleus and its central connections with the reticular formation subserve the reflex control of cardiovascular, respiratory and cardiac functions. The rostral fibres of the tract consist of gustatory fibres from the facial, glossopharyngeal and vagal nerves that project to the rostral pole of the nucleus solitarius, which is sometimes referred to as the gustatory nucleus.
The medial longitudinal fasciculus, a small, compact tract near the midline and ventral to the hypoglossal nucleus, is continuous with the ventral vestibulospinal tract. At this medullary level it is displaced dorsally by the pyramidal and lemniscal decussations. It ascends in the pons and midbrain, maintaining its relationship to the central grey matter and midline, so it is near the somatic efferent nuclear column. Fibres from a variety of sources course for short distances in the tract.
CASE 2 Avellis’ Syndrome
COMMENT: The patient demonstrated the classic features of Avellis syndrome due to a lesion (infarction) in the medial aspect of the lower medulla involving to variable extents. The neuro-anatomic structures involved include the corticospinal tracts causing contralateral hemiparesis, the medial lemniscus leading to impaired posterior column sensibility, the accessory nerve causing mild atrophy of the sternomastoid muscle, and the hypoglossal nerve producing atrophy of the tongue with fasciculations. This syndrome is rare, and variably described; in a number of cases, palatal weakness has been observed. See Figure 10.10.
Pyramidal Tract
Each pyramid contains descending corticospinal fibres, derived from the ipsilateral cerebral cortex, which have traversed the internal capsule, midbrain and pons (Fig. 10.11). Approximately 70% to 90% of the axons leave the pyramids in successive bundles, crossing in and deep to the ventral median fissure as the pyramidal decussation. In the rostral medulla, fibres cross by inclining ventromedially, whereas more caudally, they pass dorsally, decussating ventral to the central grey matter. The decussation is orderly, with fibres destined to end in the cervical segments crossing first. They continue to pass dorsally as they descend, reaching the contralateral spinal lateral funiculus as the crossed lateral corticospinal tract. Most uncrossed corticospinal fibres descend ventromedially in the ipsilateral ventral funiculus, as the ventral corticospinal tract. A minority run dorsolaterally to join the lateral corticospinal tracts as a small uncrossed component. The corticospinal tracts display somatotopy at almost all levels. In the pyramids the arrangement is like that at higher levels, in that the most lateral fibres subserve the most medial arm and neck movements. Similar somatotopy is ascribed to the lateral corticospinal tracts within the spinal cord.
Dorsal Column Nuclei
The nuclei gracilis and cuneatus are part of the pathway that is considered the major route for discriminative aspects of tactile and locomotor (proprioceptive) sensation. The upper regions of both nuclei are reticular and contain small and large multipolar neurones with long dendrites. The lower regions contain clusters of large, round neurones with short and profusely branching dendrites. Upper and lower zones differ in their connections, but both receive terminals from the dorsal spinal roots at all levels. Dorsal funicular fibres from neurones in the spinal grey matter terminate only in the superior, reticular zone. Variable ordering and overlap of terminals, on the basis of spinal root levels, occur in both zones. The lower extremity is represented medially, the trunk ventrally and the digits dorsally. There is modal specificity; that is, lower levels respond to low-threshold cutaneous stimuli, and upper reticular levels respond to inputs from fibres serving receptors in the skin, joints and muscles. The cuneate nucleus is divided into several parts. Its middle zone contains a large pars rotunda, in which rostrocaudally elongated, medium-sized neurones are clustered between bundles of densely myelinated fibres. The reticular poles of its rostral and caudal zones contain scattered but evenly distributed neurones of various sizes. The pars triangularis is smaller and laterally placed. There is a somatotopic pattern of termination of cutaneous inputs from the upper limb on the cell clusters of the pars rotunda. Terminations are diffuse in the reticular poles.
The accessory cuneate nucleus, dorsolateral to the cuneate, is part of the spinocerebellar system of precerebellar nuclei (see Fig. 10.9); it contains large neurones like those in the spinal thoracic nucleus. These form the posterior external arcuate fibres, which enter the cerebellum by the ipsilateral inferior peduncle. The nucleus receives the lateral fibres of the fasciculus cuneatus, carrying proprioceptive impulses from the upper limb (which enter the cervical spinal cord rostral to the thoracic nucleus). Its efferent fibres form the cuneocerebellar tract. A group of neurones, called nucleus Z, has been identified in animals between the upper pole of the nucleus gracilis and the inferior vestibular nucleus and is said to be present in the human medulla. Its input is probably from the dorsal spinocerebellar tract, which carries proprioceptive information from the ipsilateral lower limb, and it projects through internal arcuate fibres to the contralateral medial lemniscus.
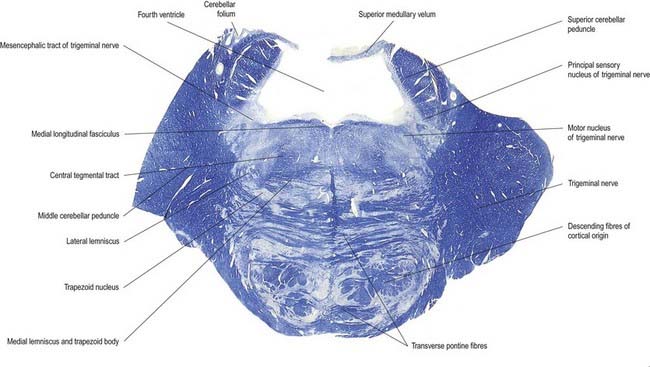
Trigeminal Sensory Nucleus
On entering the pons, the fibres of the sensory root of the trigeminal nerve run dorsomedially toward the principal sensory nucleus, which is situated at this level (Fig. 10.12). Before reaching the nucleus, approximately 50% of the fibres divide into ascending and descending branches; the others ascend or descend without division. The descending fibres, 90% of which are less than 4 µm in diameter, form the spinal tract of the trigeminal nerve, which reaches the upper cervical spinal cord. The tract embraces the spinal trigeminal nucleus (Figs 10.5, 10.6, 10.8, 10.13, 10.14). There is a precise somatotopic organization in the tract. Fibres from the ophthalmic root lie ventrolaterally, those from the mandibular root lie dorsomedially and the maxillary fibres lie between them. The tract is completed on its dorsal rim by fibres from the sensory roots of the facial, glossopharyngeal and vagus nerves. All these fibres synapse in the nucleus caudalis.
The detailed anatomy of the trigeminospinal tract excited early clinical interest because it was recognized that dissociated sensory loss could occur in the trigeminal area. For example, in Wallenberg’s syndrome (see Case 3), occlusion of the posterior inferior cerebellar branch of the vertebral artery leads to loss of pain and temperature sensation in the ipsilateral half of the face, with retention of common sensation.
The spinal nucleus is considered to consist of three parts: the subnucleus oralis (which is most rostral and adjoins the principal sensory nucleus), the subnucleus interpolaris and the subnucleus caudalis (which is the most caudal part and is continuous below with the dorsal grey column of the spinal cord). The structure of the subnucleus caudalis is different from that of the other trigeminal sensory nuclei. It has a structure analogous to that of the dorsal horn of the spinal cord, with a similar arrangement of cell laminae, and it is involved in trigeminal pain perception. Cutaneous nociceptive afferents and small-diameter muscle afferents terminate in layers I, II, V and VI of the subnucleus caudalis. Low-threshold mechanosensitive afferents of Aβ neurones terminate in layers III and IV of the subnucleus caudalis and rostral (interpolaris, oralis and main sensory) nuclei.
There are distinct subtypes of cells in lamina II. Afferents from ‘higher centres’ arborize within it, as do axons from nociceptive and low-threshold afferents. Descending influences from these higher centres include fibres from the periaqueductal grey matter and from the nucleus raphe magnus and associated reticular formation.
Vagal Nucleus
The vagal nucleus (also known as the dorsal motor nucleus of the vagus) lies dorsolateral to the hypoglossal nucleus, from which it is separated by the nucleus intercalatus. It extends caudally to the first cervical spinal segment and rostrally to the open part of the medulla under the vagal triangle (see Fig. 10.1).
Hypoglossal Nucleus
The prominent hypoglossal nucleus lies near the midline in the dorsal medullary grey matter. It is approximately 2 cm long. Its rostral part lies beneath the hypoglossal triangle in the floor of the fourth ventricle, and its caudal part extends into the closed part of the medulla (see Figs 10.2, 10.6, 5.11).
Hypoglossal fibres emerge ventrally from their nucleus, traverse the reticular formation lateral to the medial lemniscus, pass medial to (or sometimes through) the inferior olivary nucleus and curve laterally to emerge superficially as a linear series of 10 to 15 rootlets in the ventrolateral sulcus between the pyramid and olive (see Fig. 10.3).
Inferior Olivary Nucleus
The olivary nuclear complex consists of the large inferior olivary nucleus and the much smaller medial and dorsal accessory olivary nuclei. They are the so-called precerebellar nuclei, a group that also includes the pontine, arcuate, vestibular, reticulocerebellar and spinocerebellar nuclei, all of which receive afferents from specific sources and project to the cerebellum. The inferior olivary nucleus contains small neurones, most of which form the olivocerebellar tract, which emerges either from the hilum or through the adjacent wall to run medially and intersect the medial lemniscus (see Fig. 10.9). Its fibres cross the midline and sweep either dorsal to or through the opposite olivary nucleus. They intersect the lateral spinothalamic and rubrospinal tracts and the spinal trigeminal nucleus and enter the contralateral inferior cerebellar peduncle, where they constitute its major component. Fibres from the contralateral inferior olivary complex terminate on Purkinje cells in the cerebellum as climbing fibres; there is a one-to-one relationship between Purkinje cells and neurones in the complex. Afferent connections to the inferior olivary nucleus are both ascending and descending. Ascending fibres, mainly crossed, arrive from all spinal levels in the spino-olivary tracts and via the dorsal columns. Descending ipsilateral fibres come from the cerebral cortex, thalamus, red nucleus and central grey of the midbrain. In part, the two latter projections make up the central tegmental tract (fasciculus) that forms the olivary amiculum.
The medial accessory olivary nucleus is a curved grey lamina that is concave laterally and located between the medial lemniscus and pyramid and the ventromedial aspect of the inferior olivary nucleus. The dorsal accessory olivary nucleus is a similar lamina dorsomedial to the inferior olivary nucleus. Both nuclei are connected to the cerebellum. The accessory nuclei are phylogenetically older than the inferior and are connected with the palaeocerebellum. In all connections—cerebral, spinal and cerebellar—the olivary nuclei sometimes display very specific somatotopy, particularly in their cerebellar connections, which are described in detail in Chapter 13.
Nucleus Solitarius
Swallowing and Gag Reflexes
During the normal processes of eating and drinking, passage of material to the rear of the mouth stimulates branches of the glossopharyngeal nerve in the oropharynx (Fig. 10.15). This information is relayed via the nucleus solitarius to the nucleus ambiguus, which contains the motor neurones innervating the muscles of the palate, pharynx and larynx. The nasopharynx is closed off from the oropharynx by elevation of the soft palate. The larynx is raised, its entrance narrows and the glottis is closed. Peristaltic activity down the oesophagus to the stomach is mediated through the pharyngeal plexus.
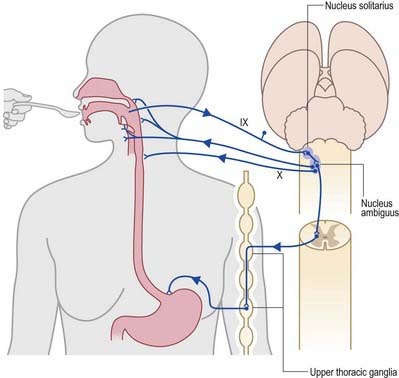
Fig. 10.15 Swallowing and gag reflexes.
(Redrawn from MacKinnon, P., Morris, J. (Eds.), 1990. Oxford Textbook of Functional Anatomy, vol 3, Head and Neck. Oxford University Press, Oxford. By permission of Oxford University Press.)
Nucleus Ambiguus
Cough and Sneeze Reflexes
A similar mechanism underlies sneezing (Fig. 10.16), except that the stimulus arises from the nasal mucosa, and afferent impulses are conveyed by the ophthalmic or maxillary divisions of the trigeminal nerve to the trigeminal sensory nucleus. After sharp inhalation, explosive exhalation occurs, with closure of the oropharyngeal isthmus by action of the palatoglossus, which diverts air through the nasal cavity and expels the irritant.
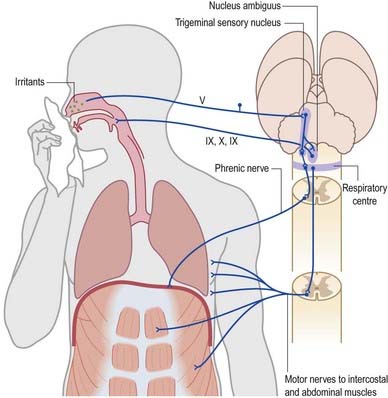
Fig. 10.16 Sneeze and cough reflexes.
(Redrawn from MacKinnon, P., Morris, J. (Eds.), 1990. Oxford Textbook of Functional Anatomy, vol 3, Head and Neck. Oxford University Press, Oxford. By permission of Oxford University Press.)
Pons
External Features and Relations
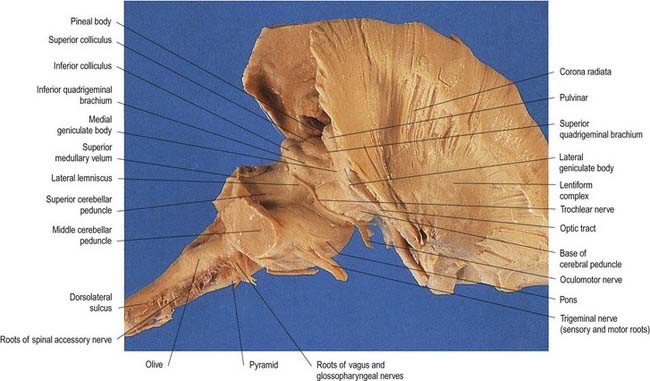
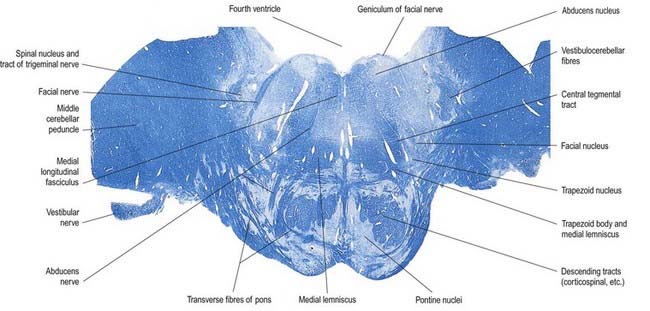
The pons lies rostral to the medulla and caudal to the midbrain. Ventrally, the site of transition with the medulla is demarcated superficially by a transverse sulcus. Laterally, in a region known as the cerebellopontine angle (see Figs 10.2, 10.3), the facial, vestibulocochlear and glossopharyngeal roots and the nervus intermedius all lie on the choroid plexus of the fourth ventricle (which protrudes from the foramen of Luschka into the subarachnoid space). The ventral surface of the pons (see Fig. 10.3) is separated from the clivus (basisphenoid and dorsum sellae) by the cisterna pontis. It is markedly convex transversely and less so vertically; it grooves the petrous part of the temporal bone laterally up to the internal acoustic meatus. The surface has a shallow vertical median sulcus in which the basilar artery runs, bounded bilaterally by prominences that are formed partly by underlying corticospinal fibres as they descend through the pons. Bundles of transverse fibres, bridging the midline and originating from nuclei in the basal pons (nuclei pontis), converge on each side into the large middle cerebellar peduncle and project to the cerebellum. The trigeminal nerve emerges near the mid-pontine level. It has a small superomedial motor root and a large inferolateral sensory root.
The dorsal surface of the pons is hidden by the cerebellum, which covers the rostral half of the rhomboid fossa, into which the aqueduct of the midbrain empties. The roof of the fossa is formed by a thin sheet of tissue, the superior medullary velum, and is overlain by the lingula of the vermis of the cerebellum. The velum is attached on each side to the superior cerebellar peduncles and is enclosed by pia mater above and ependyma below (see Fig. 5.11). The abducens nerves decussate in the velum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree