Chapter 15 Diencephalon
The diencephalon is part of the prosencephalon (forebrain), which develops from the foremost primary cerebral vesicle and differentiates into a caudal diencephalon and rostral telencephalon. The cerebral hemispheres develop from the sides of the telencephalon, each containing a lateral ventricle. The sites of evagination become the interventricular foramina, through which the two lateral ventricles and midline third ventricle communicate. The diencephalon corresponds largely to the structures that develop lateral to the third ventricle.
The lateral walls of the diencephalon form the epithalamus most superiorly, the thalamus centrally and the subthalamus and hypothalamus most inferiorly. The epithalamus in the mature brain contains the anterior and posterior paraventricular nuclei, the medial and lateral habenular nuclei, the stria medullaris thalami and the pineal gland. The thalamus undergoes proliferation to form numerous nuclear masses that have extensive reciprocal connections with the cerebral cortex. The subthalamic region consists of the subthalamic nucleus, zona incerta and fields of Forel. The subthalamic nucleus is closely related to the basal ganglia and is considered with them in Chapter 14. The hypothalamic rudiment gives rise to most of the subdivisions of the adult hypothalamus.
Thalamus
The thalamus is an ovoid nuclear mass, approximately 4 cm long, that borders the dorsal part of the third ventricle (Figs 15.1–15.3; see also Fig. 1.10). The narrow anterior pole lies close to the midline and forms the posterior boundary of the interventricular foramen. Posteriorly, an expansion, the pulvinar, extends beyond the third ventricle to overhang the superior colliculus (Fig. 15.4). The brachium of the superior colliculus (superior quadrigeminal brachium) separates the pulvinar above from the medial geniculate body below. A small oval elevation, the lateral geniculate body, lies lateral to the medial geniculate.
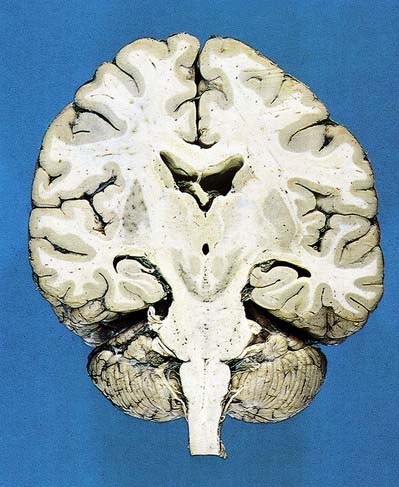
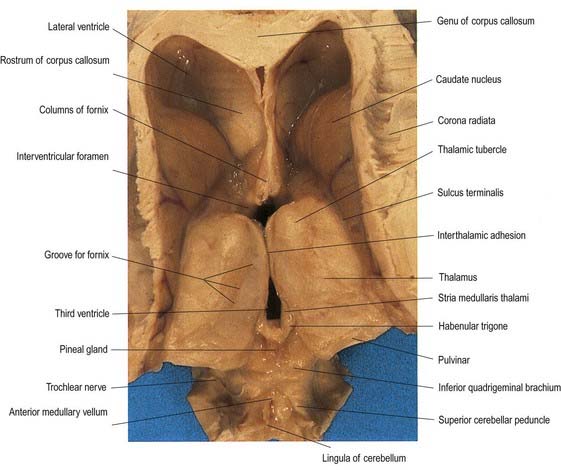
Fig. 15.1 Dorsal half of a brain sectioned in an oblique coronal plane that passes through the cerebral hemispheres, diencephalon, midbrain, pons and medulla oblongata, to show the general disposition of the main structures, some of which are labelled in Figure 15.3. Compare also with Figure 1.10.
(Photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
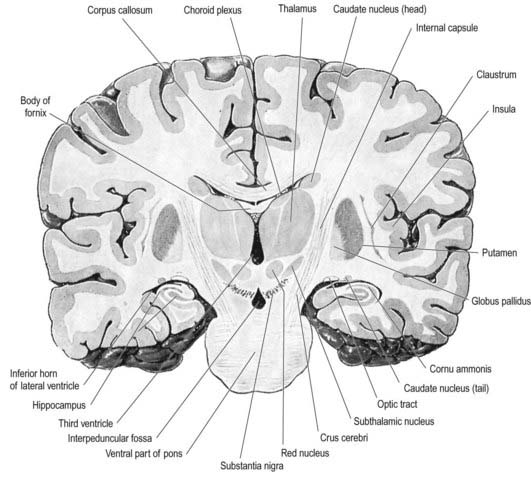
Fig. 15.3 Coronal section of the brain showing the principal parts of the diencephalon and basal ganglia. Compare with Figure 1.10.
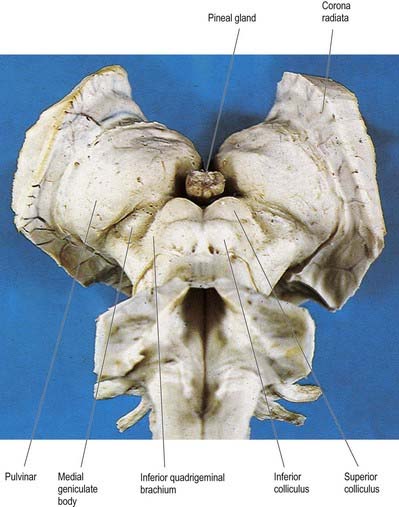
Fig. 15.4 Oblique view of the dorsal aspect of the brain stem and thalamus.
(Photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
The superior (dorsal) surface of the thalamus (see Fig. 15.2) is covered by a thin layer of white matter, the stratum zonale. It extends laterally from the line of reflection of the ependyma (taenia thalami) and forms the roof of the third ventricle. This curved surface is separated from the overlying body of the fornix by the choroid fissure, with the tela choroidea within it. More laterally, it forms part of the floor of the lateral ventricle. The lateral border of the superior surface of the thalamus is marked by the stria terminalis and the overlying thalamostriate vein, which separate the thalamus from the body of the caudate nucleus. Laterally, a slender sheet of white matter, the external medullary lamina, separates the main body of the thalamus from the reticular nucleus. Lateral to this, the thick posterior limb of the internal capsule lies between the thalamus and the lentiform complex.
The medial surface of the thalamus is the superior (dorsal) part of the lateral wall of the third ventricle (see Fig. 5.8). It is usually connected to the contralateral thalamus by an interthalamic adhesion behind the interventricular foramina. The boundary with the hypothalamus is marked by an indistinct hypothalamic sulcus, which curves from the upper end of the cerebral aqueduct to the interventricular foramen. The thalamus is continuous with the midbrain tegmentum, the subthalamus and the hypothalamus.
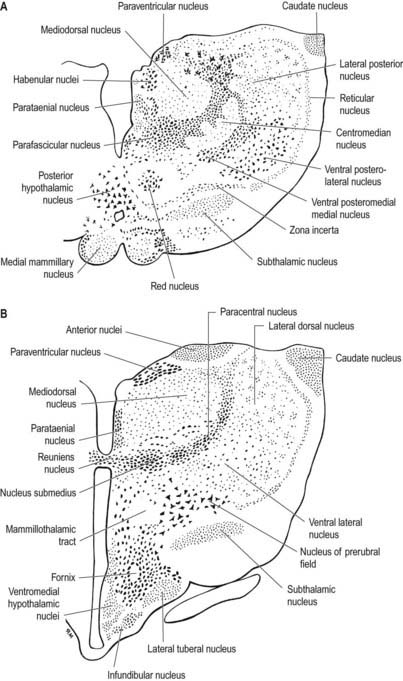
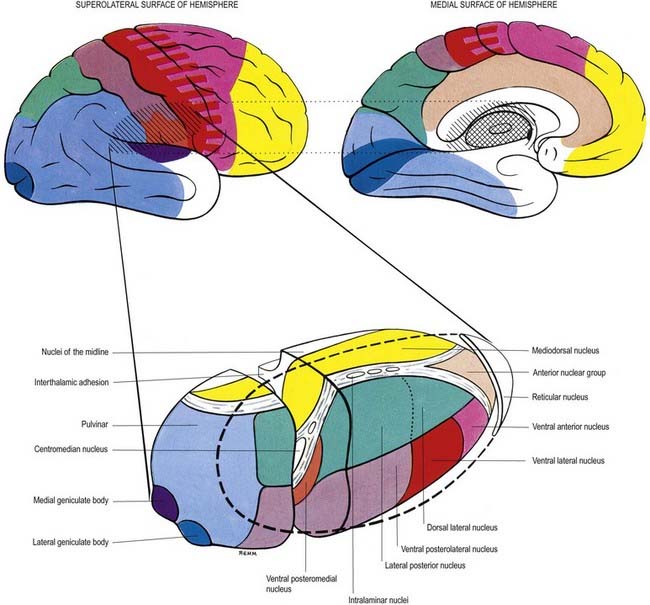
Internally, the thalamus is divided into anterior, medial and lateral nuclear groups by a vertical Y-shaped sheet of white matter, the internal medullary lamina (Figs 15.5, 15.6). In addition, intralaminar nuclei lie embedded within, and surrounded by, the internal medullary lamina. Midline nuclei either abut the ependyma of the lateral walls of the third ventricle medially or lie adjacent to, and to some extent within, the interthalamic adhesion. Reticular nuclei lie lateral to the main nuclear mass, separated from it by the external medullary lamina.
In general, thalamic nuclei both project to and receive fibres from the cerebral cortex (see Fig. 15.6). The whole cerebral cortex, not only the neocortex but also the phylogenetically older palaeocortex of the piriform lobe and archicortex of the hippocampal formation, are reciprocally connected with the thalamus. The thalamus is the major route by which subcortical neuronal activity influences the cerebral cortex, and the greatest input to most thalamic nuclei comes from the cerebral cortex.
Anterior Group of Thalamic Nuclei
The anterior group of nuclei are enclosed between the arms of the Y-shaped internal medullary lamina and underlie the anterior thalamic tubercle (see Fig. 15.2, 15.6). Three subdivisions are recognized. The largest is the anteroventral nucleus; the others are the anteromedial and anterodorsal nuclei.
The cortical targets of efferent fibres from the anterior nuclei of the thalamus lie largely on the medial surface of the hemisphere (see Fig. 15.6). They include the anterior limbic area (in front of and inferior to the corpus callosum), the cingulate gyrus and the parahippocampal gyrus (including the medial entorhinal cortex and the pre- and para-subiculum). These thalamocortical pathways are reciprocal. There also appear to be minor connections between the anterior nuclei and the dorsolateral prefrontal and posterior areas of the neocortex. The anterior thalamic nuclei are believed to be involved in the regulation of alertness and attention and in the acquisition of memory.
Medial Group of Thalamic Nuclei
The single component of this thalamic region is the mediodorsal or dorsomedial nucleus, which is particularly large in humans. Laterally, it is limited by the internal medullary lamina and intralaminar nuclei (see Figs. 15.5, 15.6). Medially, it abuts the midline parataenial and reuniens (medioventral) nuclei. It can be divided into anteromedial magnocellular and posterolateral parvocellular parts.
The larger posterolateral parvocellular division connects reciprocally with the dorsolateral and dorsomedial prefrontal cortex, the anterior cingulate gyrus and the supplementary motor area. In addition, efferent fibres pass to the posterior parietal cortex.
Lateral Group of Thalamic Nuclei
The lateral nuclear complex, lying lateral to the internal medullary lamina, is the largest major division of the thalamus (see Fig. 15.6). It is divided into dorsal and ventral tiers of nuclei. The lateral dorsal nucleus, lateral posterior nucleus and pulvinar all lie dorsally. The lateral and medial geniculate nuclei lie inferior to the pulvinar, near the posterior pole of the thalamus. The ventral tier nuclei are the ventral anterior, ventral lateral and ventral posterior nuclei.
Ventral Anterior Nucleus
The ventral anterior (VA) complex lies at the anterior pole of the ventral nuclear group. It is limited anteriorly by the reticular nucleus and posteriorly by the ventral lateral nucleus, and it lies between the external and internal medullary laminae. It consists of a principal part (VApc) and a magnocellular part (VAmc). The subcortical connections to this region are largely ipsilateral from the internal segment of the globus pallidus and the pars reticulata of the substantia nigra. The terminal fields from these origins do not overlap. Fibres from the globus pallidus end in VApc. The substantia nigra projects to VAmc. Corticothalamic fibres from premotor cortex (area 6) terminate in VApc, and fibres from the frontal eye field (area 8) terminate in VAmc. The VA thalamus does not appear to receive fibres directly from the motor cortex. The efferent projections from VA are incompletely known. Some pass to intralaminar thalamic nuclei, and others project to widespread regions of the frontal lobe and to the anterior parietal cortex. Their functions are unclear. The VA thalamus appears to play a central role in the transmission of the cortical ‘recruiting response,’ a phenomenon in which stimulation of the thalamus can initiate long-lasting, high-voltage repetitive negative electrical waves over much of the cerebral cortex.
Ventral Posterior Nucleus
There is a well-ordered topographical representation of the body in the VP nucleus. The VPl is organized so that sacral segments are represented laterally and cervical segments medially. The latter abut the face area of representation (trigeminal territory) in VPm. Taste fibres synapse anteriorly and ventromedially within the VPl nucleus.
Medial Geniculate Nucleus
The medial geniculate nucleus, which is a part of the auditory pathway (Ch. 12), is located within the medial geniculate body, a rounded elevation situated posteriorly on the ventrolateral surface of the thalamus and separated from the pulvinar by the superior quadrigeminal brachium. It receives fibres travelling in the inferior quadrigeminal brachium. Three major subnuclei—medial, ventral and dorsal—are recognized within it. The inferior brachium separates the medial (magnocellular) nucleus, which consists of sparse, deeply staining neurones, from the lateral nucleus, which is made up of medium-sized, densely packed and darkly staining cells. The dorsal nucleus overlies the ventral nucleus and expands posteriorly; therefore, it is sometimes known as the posterior nucleus of the medial geniculate. It contains small to medium-sized, pale-staining cells, which are less densely packed than those of the lateral nucleus. The ventral nucleus receives fibres from the central nucleus of the ipsilateral inferior colliculus via the inferior quadrigeminal brachium and also from the contralateral inferior colliculus. The nucleus contains a complete tonotopic representation. Low-pitched sounds are represented laterally, and progressively higher-pitched sounds are encountered as the nucleus is traversed from lateral to medial. The dorsal nucleus receives afferents from the pericentral nucleus of the inferior colliculus and from other brain stem nuclei of the auditory pathway. A tonotopic representation has not been described in this subdivision, and cells within the dorsal nucleus respond to a broad range of frequencies. The magnocellular medial nucleus receives fibres from the inferior colliculus and from the deep layers of the superior colliculus. Neurones within the magnocellular subdivision may respond to modalities other than sound. However, many cells respond to auditory stimuli, usually to a wider range of frequencies than do neurones in the ventral nucleus. Many units show evidence of binaural interaction, with the leading effect arising from stimuli in the contralateral cochlea. The ventral nucleus projects primarily to the primary auditory cortex. The dorsal nucleus projects to auditory areas surrounding the primary auditory cortex. The magnocellular division projects diffusely to auditory areas of the cortex and to adjacent insular and opercular fields.
Lateral Geniculate Nucleus
The lateral geniculate body, which is part of the visual pathway (Ch. 12), is a small ovoid ventral projection from the posterior thalamus (Fig. 15.7). The superior quadrigeminal brachium enters the posteromedial part of the lateral geniculate body dorsally, lying between the medial geniculate body and the pulvinar.
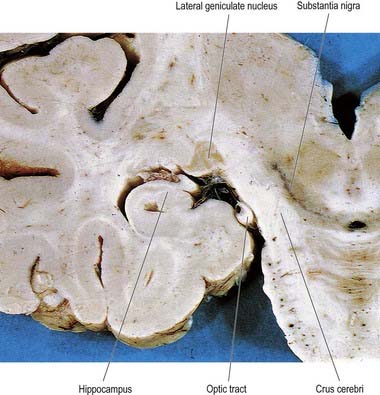
Fig. 15.7 Coronal section through the brain showing the lateral geniculate nucleus.
(Photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
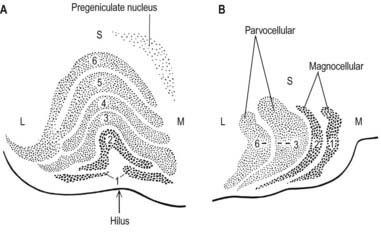
The lateral geniculate nucleus is an inverted, somewhat flattened U-shaped nucleus and is laminated. Its internal organization is usually described on the basis of six laminae, although seven or eight may be present. The laminae are numbered 1 to 6, from the innermost ventral to the outermost dorsal (Fig. 15.8). Laminae 1 and 2 consist of large cells, the magnocellular layers, whereas layers 4 to 6 have smaller neurones, the parvocellular laminae. The apparent gaps between laminae are called the interlaminar zones. Most ventrally, an additional superficial, or S, lamina is recognized.
The lateral geniculate nucleus is organized in a visuotopic manner and contains a precise map of the contralateral visual field. The vertical meridian is represented posteriorly, the peripheral anteriorly, the upper field laterally, and the lower field medially (Ch. 12). Similar precise point-to-point representation is also found in the projection of the lateral geniculate nucleus to the visual cortex. Radially arranged inverted pyramids of neurones in all laminae respond to a single small area of the contralateral visual field and project to a circumscribed area of cortex. The termination of geniculocortical axons in the visual cortex is considered in detail in Chapter 16.
Intralaminar Nuclei
The intralaminar nuclei are collections of neurones within the internal medullary lamina of the thalamus. Two groups of nuclei are recognized. The anterior (rostral) group is subdivided into central medial, paracentral and central lateral nuclei. The posterior (caudal) intralaminar group consists of the centromedian and parafascicular nuclei. The designations central medial and centromedian can lead to confusion, but they are an accepted part of the terminology of thalamic nuclei in common usage. The centromedian nucleus is much larger, is considerably expanded in humans in comparison with other species and is importantly related to the globus pallidus, deep cerebellar nuclei and motor cortex. Anteriorly, the internal medullary lamina separates the mediodorsal nucleus from the ventral lateral complex. It is occupied by the paracentral nucleus laterally and the central medial nucleus ventromedially, as the two laminae converge toward the midline. A little more posteriorly, the central lateral nucleus appears dorsally in the lamina as the latter splits to enclose the lateral dorsal nucleus. More posteriorly, at the level of the ventral posterior nucleus, the lamina splits to enclose the ovoid centromedian nucleus. The smaller parafascicular nucleus lies more medially.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree