Fig. 14.1
Herniation syndromes
Uncal herniation occurs when a lateral hemisphere mass displaces the medial edge of the uncus and hippocampal gyrus through the tentorium. Initially there is dilation of the ipsilateral pupil due to compression of the third nerve followed by ipsilateral hemiparesis from compression of the cerebral peduncle against the tentorial edge. Compression of the posterior cerebral artery may occur with ischemia/infarction of the ipsilateral occipital lobe producing a contralateral homonymous hemianopia. The late events are similar to that of central herniation.
Tonsillar herniation is due to compression of the cerebellar tonsils against the medulla producing early nuchal rigidity and head tilt followed by coma and respiratory arrest.
Depending on the tumor’s rate of growth, death can occur as early as the tumor reaches the size of about 100 g (~ 1 × 1011 cells) or about the size of a golf ball. This is compared to systemic tumors where death occurs typically when the tumor reaches about 1000 g. Slow growing CNS tumors can grow considerably larger before herniation develops.
Cerebral Edema
Cerebral edema, excess fluid either locally or diffusely in the brain, develops as a result of many pathological processes including brain tumors, head trauma, brain abscess and meningitis, anoxia, and some metabolic disorders. Cerebral edema has been divided into three types: vasogenic, cytotoxic, and interstitial. Interstitial edema is uncommon and results in obstructive hydrocephalus with CSF backing into the white matter around ventricles .
Vasogenic edema is the most common form of cerebral edema and frequently surrounds brain tumors. The edema results from the tumor secreting proteases causing localized dysfunction of the blood–brain barrier with increased permeability of capillary endothelial cells. Vasogenic edema develops mainly in cerebral white matter and uncommonly in gray matter. Vasogenic edema from a brain tumor is reduced following administration of corticosteroids presumably because steroids restore the blood brain barrier. Unfortunately, steroids seldom improve cerebral edema following cerebral infarction or head trauma. Interestingly, hypoalbuminemia and increased systemic venous pressure cause peripheral edema but not edema in the brain.
Cytotoxic edema develops from a fluid swelling of neurons, glia, and endothelial cells usually following hypoxia. Hypoxia causes failure of the ATP-dependent sodium pump within cells with subsequent accumulation of intracellular sodium and water. Cytotoxic edema causes a reduction in the extracellular fluid space so there is little increase in the mass effect. Brain tumors cause mainly vasogenic edema while infarctions cause both cytotoxic and vasogenic edema. Cytotoxic edema is difficult to reduce and does not respond to corticosteroids. Characteristics of vasogenic and cytotoxic edema are listed in Table 14.1 .
Table 14.1
Features of vasogenic and cytotoxic brain edema
Feature | Vasogenic | Cytotoxic |
|---|---|---|
Pathogenesis | Opening of blood–brain barrier with increased capillary permeability | Cellular swelling of glia, neurons, and endothelial cells from hypoxia |
Edema location | Chiefly white matter | Gray and white matter |
Edema fluid composition | Plasma filtrate | Increased intracellular water and sodium |
Common causes | Brain tumor, abscess, infarction, trauma, lead encephalopathy, hemorrhages, and bacterial meningitis | Brain hypoxia/ischemia |
Corticosteroid effect | Reduces edema | No edema reduction |
Osmotic agents | Acutely reduces water in normal brain | Acutely reduces water in edematous brain |
Malignant Gliomas
Introduction
Malignant gliomas account for 70 % of new cases malignant primary brain tumors in adults each year. The tumor tends to occur in older adults (mean age 55 years). No underlying cause has been identified for the majority of malignant gliomas with the only established risk factor being exposure to ionizing radiation. The prognosis for malignant gliomas is poor with no adequate treatment currently available.
Pathophysiology
Grade 1 astrocytomas are slow growing, have a normal cellular morphology, and do not induce abnormal vascularity. The most benign astrocytomas typically arise in the optic nerve or brainstem and patients often survive decades after diagnosis.
Malignant glioma or glioblastoma multiforme usually arises in the cerebral hemispheres and appears grossly as a soft relatively circumscribed mass that may contain cysts or necrosis (Fig. 14.2). The tumor extends many centimeters beyond the apparent gross or neuroimaging margin. Microscopically, the tumor is highly cellular containing heterogeneous, extremely pleomorphic cell types. Multinucleated giant cells are frequently seen. There is extensive neovascularization with marked proliferation of endothelium of small capillaries feeding the tumor. Gliomas rarely metastasize outside the brain.
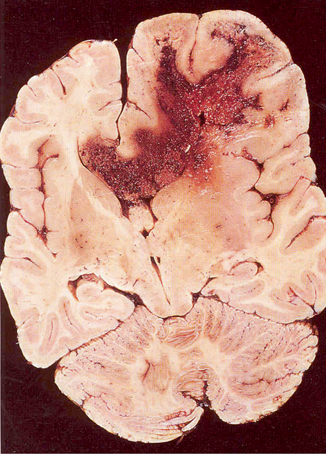
Fig. 14.2
Pathology, glioblastoma multiforme with cysts and necrosis and extension into the corpus collosum. (Courtesy of Dr. Mark Becher)
Malignant gliomas are characterized by self-initiation, uncontrolled proliferation, evasion of apoptosis, avoidance of immune surveillance, increased angiogenesis, necrosis, and diffuse tumor cell invasion into adjacent normal brain. Common genetic alterations common in malignant gliomas include amplification and/or over expression of receptor tyrosine kinases and angiogenesis factors. Epidermal growth factor receptor (EGFR) gene amplification is the most prominent mutated receptor tyrosine kinase receptor. Platelet-derived growth factor (PDGF) and its receptors are important in angiogenesis and tumor growth in gliomas . The tumor-suppressor phosphatase and tensin homolog (PTEN), which normally is important for suppressing tumors, can be removed when chromosome 10 is deleted in some malignant gliomas. The exact cell origin of gliomas is not known but there is increasing interest in glioma stem cells as the potential genesis. The hypothesis is that the normal stem cell becomes malignant and replicates producing tumor cells even though the pool of stem cells in the tumor remains small. The abnormal stem cells maintain their stem cell properties and are relatively resistant to chemotherapy and radiotherapy and induce angiogenesis.
Major Clinical Features
Early signs and symptoms include headaches in 50 %, altered mental status with confusion or memory loss in 50 %, hemiparesis in 40 %, aphasia in 15 %, visual field loss in 5 %, and seizures in 20 %. The seizures may be either focal seizures (usually motor) or secondarily generalized tonic-clonic seizures. The classic headache is a morning severe headache that can awaken the patient but often the headache is similar in character to a tension headache . Papilledema may be seen on fundoscopy. In the absence of treatment, symptoms of glioblastoma progress relatively rapidly over weeks.
Major Laboratory Findings
MRI with gadolinium is the test of choice. On MRI, the tumor characteristically has low signal intensity on T1-weighted and high signal intensity on T2-weighted images (Fig. 14.3). The appearance of contrast-enhanced T1-weighted images is that of a heterogeneous central low signal surrounded by a ring of high intensity enhancement. Areas of central necrosis are common. Surrounding the high intensity ring are hypointense signals representing cerebral edema and tumor infiltration. The extent of cerebral edema varies from tumor to tumor. The CT scan shows variably hypodense or isodense lesions surrounded by hypodense cerebral edema.
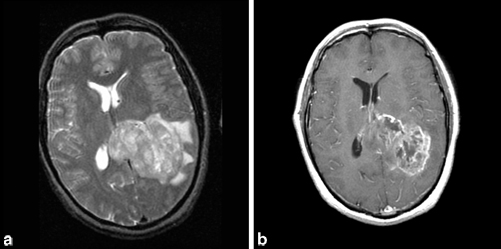
Fig. 14.3
a Axial, T2-weighted MRI showing left parietal glioblastoma multiforme extending into corpus collosum with surrounding vasogenic edema and causing midline shift. b Axial, T1-weighted MRI with gadolinium showing enhancement of glioblastoma multiforme. (Courtesy of Dr. Blaine Hart)
The EEG shows focal or extensive slowing (delta waves) in the region of the tumor with varying degrees of spikes at the tumor edge and surrounding edematous brain.
Principles of Management and Prognosis
Glioblastoma multiforme (WHO grade IV) is always fatal. Thus, palliative management aims at slightly prolonging survival and controlling symptoms. Corticosteroids reduce the surrounding edema and temporarily improve symptoms, prolonging survival for 1–3 months. Following steroids, the headache lessens, the mental status perks up, and focal neurologic signs (such as hemiparesis) improve. Since steroids do not affect tumor growth, the signs and symptoms eventually return. Neurologic side effects of high dose corticosteroids include psychosis, hyperactivity, irritability, insomnia , and myopathy.
Surgical removal of the main tumor mass (debulking) only slightly improves the length of survival but often temporarily improves neurologic signs by reducing intracranial pressure. Since fingers of the tumor have already spread far beyond the main tumor margins, debulking is never curative. However, it does allow for a histologic diagnosis and molecular studies. Brain biopsy and minor tumor removal have no effect on survival.
Radiotherapy after surgery only slightly improves survival compared to surgery alone because malignant gliomas are highly resistant to radiotherapy. Side effects of the radiotherapy commonly include anorexia, nausea, hair loss, and fatigue and may include late radiation necrosis of normal brain.
The value of many chemotherapeutic agents for gliomas is controversial. The choice of an agent is difficult as many antineoplastic agents do not cross the blood–brain barrier and thus poorly penetrate the tumor. However, temozolomide along with radiotherapy in patients whose tumor has epigenetic silencing of the O6-methylguanine-DNA methyltransferase (MGMT) gene do benefit and show a median survival of 22 months. MGMT promoter methylation (present in 40 % of malignant gliomas) silences the gene and decreases DNA repair activity which heightens tumor cells susceptibility to temozolomide. Unfortunately, the mean time to tumor recurrence is only 7 months.
In summary, if the malignant glioma (WHO grade IV) is untreated, the mean survival from diagnosis is less than 6 months and few adult patients survive longer than 18 months. For previously healthy adult patients receiving corticosteroids, surgical tumor debulking and radiotherapy, 40 % survive one year, 10 % survive 2 years, and < 1 % survive 5 years. The prognosis for anaplastic astrocytoma (WHO grade III) is 2–3 years and for low grade astrocytoma (WHO grade II) is 5–10 years. Poor prognostic factors include old age and the presence of many clinical signs.
Palliative care minimizes the patient’s discomfort and disability. Headaches can be controlled with surgical debulking, corticosteroids, and analgesics. Anticonvulsants should be given to patients who experience seizures. Cognitive dysfunction can arise from tumor progression, effects of radiotherapy and chemotherapy, corticosteroids, metabolic disturbances, and depression. Treatment efforts should be aimed at the appropriate causes.
Meningioma
Introduction
Meningiomas are the most common adult brain tumor accounting for 30 % of all primary brain tumors. They belong to a group of brain tumors that are often called “benign” since they are slow growing, do not invade surrounding structures, and are not histologically malignant. Other common benign tumors include pituitary adenomas , acoustic neuromas, and epidermoid cysts. Meningiomas are usually solitary and only rarely metastasis inside or outside the skull. Meningiomas have a prevalence of 97 per 100,000 population with 175,000 adults currently diagnosed with the tumor. The peak incidence is in older adults. Meningiomas are located outside the brain, occur twice as often in women as men, and 3 % are incidental findings at autopsy. Table 14.2 lists their most common locations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








