Fig 10.1
Pathologic specimen showing MS plaques ( arrows) (Courtesy of Mario Kornfeld, MD)
The main physiologic effect of demyelination is to impede saltatory electrical conduction of nerve impulses jumping from one node of Ranvier where sodium channels are concentrated to the next node. Normally, there are only rare sodium channels along the myelinated axon, so conduction does not proceed in the demyelinated axonal segment. Clinical recovery of the lost function develops because new sodium channels appear along the length of the demyelinated axon allowing continuous conduction to occur (Fig. 10.2). While the conduction velocity of the electrical signal becomes slower in the axon, the signal connects to its intended target allowing function to return. The functioning of the naked axon segment is sensitive to body temperature elevations as low as 0.5 ° C, which results in conduction blockage. This accounts why patients often get a transient return of the symptoms when they develop a fever.
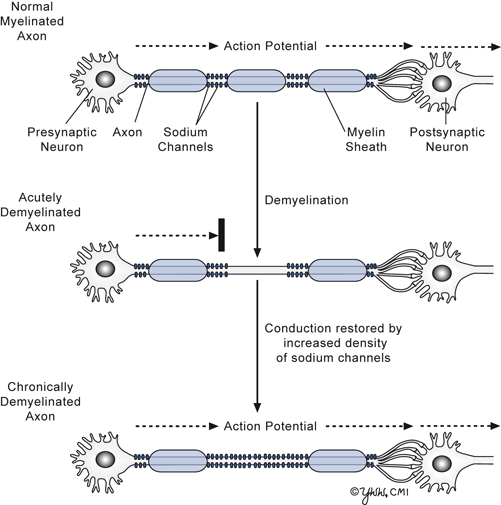
Fig 10.2
Axonal changes in acute and chronic demyelination
Over time, usually years, the plaque changes character. The relative abundance of naked axons disappears and marked gliosis develops, producing patches of hardened translucent tissue distributed randomly throughout the CNS neuroaxis along with secondary atrophy of the brain.
Plaques may occur anywhere in the CNS but not in the PNS. Common locations for plaques include the white matter of optic nerves, and white matter adjacent to lateral ventricles, corpus callosum, brainstem, cerebellum, and spinal cord. Lesions involve both hemispheres and distribute asymmetrically. Recent studies suggest that there may be several pathological forms of MS. The most common forms (> 90 %) appear to have primary damage to foci CNS myelin while the less common forms appear to have slower primary killing the oligodendrocytes without much inflammation and have a clinical history suggestive of primary progressive MS.
The cause of MS remains mysterious. Extensive searches for an infectious agent, specific myelin antigen, or genetic cause have yet to identify a likely etiology. Nevertheless, CD4 immune lymphocytes appear to play a role in the pathogenesis since most of the successful drugs to prevent MS attacks impair T cell functions.
Major Clinical Features
The clinical features and rate of MS progression vary considerably from patient to patient. Neuroimaging has shown that plaques often appear without producing clinical signs, suggesting that the demyelination does not stop axon conduction through the plaque, the plaque is in a silent brain area, or that there are alternate conduction pathways that maintain functional connectivity. In the early phase of MS, clinically apparent attacks develop about 0.3 times a year. The onset occurs over 1–3 days and does not have an identifiable trigger. Common clinical signs occur from damage to long CNS myelinated tracts. Thus, MS patients often develop hemiparesis or monoparesis (corticospinal tract), unilateral visual loss (optic nerve), sensory loss (posterior columns or spinothalamic tracts), ataxia (cerebellum or cerebellar pathways), and neurogenic bladder or paraparesis (spinal cord). Dementia , aphasia, and seizures (signs of grey matter disease) are uncommon.
Spontaneous clinical return of function usually occurs within a month. In the relapsing remitting form of MS, good or full return of function prevails but over time, attacks may leave some permanent dysfunction (Fig. 10.3). Return of clinical function occurs when the demyelinated portion of the axon converts from permitting only saltatory conduction to an axon segment that has continuous conduction (Fig. 10.3). Permanent loss of function is associated with loss of the underlying axons.
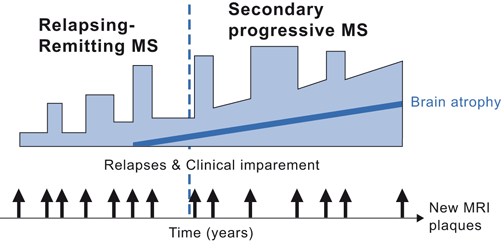
Fig 10.3
Natural history of MS
Major Laboratory Findings
There is no specific diagnostic test for multiple sclerosis. However, there are characteristic cerebrospinal fluid (CSF) changes that occur in most patients. The CSF usually shows a mild increase in total protein, an increased IgG synthesis rate (IgG index) and several oligoclonal bands seen in CSF but not blood. This indicates migration of B lymphocytes and plasmacytes from blood to brain plaques with subsequent local homogenous antibody production that then leaks into CSF. It is not known what antigen the MS antibody is directed against. The CSF may contain a small number of lymphocytes but should have a normal glucose level. Routine blood tests are normal.
MRI scans are sensitive, but not specific, indicators for myelin plaques. T2-weighted MRI lesions reflect inflammation, edema, demyelination, and gliosis. T1-weighted lesions (“black holes”) often reflect marked axonal loss in the plaque (Fig. 10.4). Gadolinium-enhancing lesions on T1-weighted images suggest disruption in the blood–brain barrier from active inflammation and demyelination that can persist up to several months. Neuroimaging lesions are commonly seen as perpendicular ovals in the white matter around the lateral ventricles, corpus callosum, cerebellum, and spinal cord, and correlate with plaques found at autopsy.
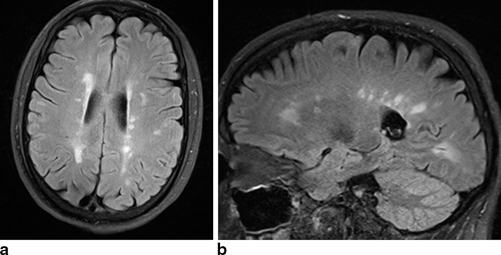
Fig 10.4
T2 FLAIR MRI scans in 35-year-old woman with MS showing a in axial view, bright lesions adjacent to the ventricles and b in sagittal view, bright lesions in corpus collosum (Courtesy of Blaine Hart, MD)
MS is a clinical diagnosis with laboratory support. The definition of MS requires dissemination of CNS white matter lesions in time (multiple attacks) and space (involving different areas of CNS white matter). Since the advent of MRI neuroimaging, it is now possible to make the diagnosis after the first clinical attack (clinically isolated syndrome) by identifying more than one lesion at 2 or more characteristic sites in brain and finding a new MRI plaque on a follow-up MRI anytime afterwards. The clinical and neuroimaging diagnosis is supported by the presence of CSF oligoclonal bands and increased IgG synthesis. No other diagnosis for the clinical signs should be apparent such as neuromyelitis optica, CNS vasculitis, and systemic lupus erythematosus.
Principles of Management and Prognosis
Treatment of MS is divided into treatment of acute exacerbations, rehabilitation of the patient, and prevention of future plaques. Acute relapses are often treated with short courses of high-dose corticosteroids (IV methylprednisolone or oral prednisone). While spontaneous clinical recovery usually takes about 4 weeks, these drugs appear to shorten the time to recovery by 1–2 weeks. However, steroids do not improve the extent of recovery or change the course of disease. Chronic treatment with steroids has not been shown to prevent subsequent relapses.
There are several standard drugs that have proven efficacy in reducing the frequency of new lesions in relapsing remitting MS. Interferon beta-1b, interferon beta-1a, and glatiramer acetate all reduce the frequency of relapses by about 30 %. Serial neuroimaging studies show these drugs reduce new T2-weighted lesions by about 60 %. These drugs have shown some effect in delaying progression of disability. The mechanisms by which interferon and glatiramer acetate work are uncertain but studies suggest the drugs affect the immune-mediated attack on the white matter. Both the interferons and glatiramer acetate require daily or weekly injections, have a moderate number of local and systemic side effects, and have some expensive (about $ 10,000/year). It is currently unknown how long these drugs should be taken.
In the past few years, new drugs have been FDA approved for MS and several additional ones are in the pipeline. Natalizumab is a humanized monoclonal antibody that antagonizes α 4-integrin of the adhesion molecule very late activating antigen (VLA-4) on leukocytes. Inhibition of VLA-4 is responsible for blockade of T cells across the blood–brain barrier . This drug is administered intravenously every 4 weeks and appears more effective than the standard anti-MS drugs. There is a 50–60 % reduction in relapses and a 90 % reduction in gadolinium-enhancing lesion on MRI compared to placebo. Unfortunately, natalizumab carries a small risk that is associated with progressive multifocal leukoencephalopathy (PML) that is usually fatal in individuals.
Fingolimod is an orally administered immunomodulator that becomes phosphorylated to act on the sphingosine-1-phosphate (SIP) receptor. The SIP receptor is responsible for lymphocytes release from lymphoid organs and thus reduces both CD4+ and CD8+ T lymphocytes in the blood. The drug also crosses the blood–brain barrier and reaches SIP receptors on oligodendrocytes, astrocytes, microglia, and neurons and thus may also have a direct neuroprotective effect on the acute plaque. Fingolimod appears to be superior to the standard anti-MS drugs reducing the annualized relapse rate from 0.33 for interferon to 0.16. Similar reductions in gadolinium-enhancing plaques were also shown. This drug has considerable potential systemic side effects requiring special monitoring when the drug is started.
A recent FDA approved drug for MS is teriflunomide, a selective inhibitor of de novo pyrimidine synthesis, which exerts a cytostatic effect on proliferating T and B lymphocytes in the periphery. It is particularly efficient in inhibiting T-cell dependent antibody production. In MS trials, the drug reduced the annualized relapse rate to 0.34 compared to 0.54 in the placebo group.
All new FDA-approved drugs for MS have shown superior efficacy in preventing both clinical relapses and new gadolinium-enhancing lesions on MRI. However, the long-term adverse effects of these drugs are unknown and their potential adverse effects are of some concern. The new drugs are very expensive (wholesale price of fingolimod is $ 50,000/year) and require considerable additional laboratory tests when administered. As such, many clinicians are reserving the newer drugs for patients in which the standard anti-MS drugs fail.
Rehabilitation aims at maximizing patient functioning. Patients may become depressed requiring counseling and antidepressant medication. Fatigue becomes a problem and is difficult to treat. Bladder spasticity with urinary incontinence may develop requiring treatment. Ataxia and spasticity affect gait, balance, and coordination interfering with activities of daily living and are difficult to treat.
After 5–15 years, relapsing-remitting patients often develop a slowly progressive illness called secondary progressive MS (Fig. 10.3). These patients enter a phase of slow steady or mildly fluctuating deterioration of neurologic function that is often attributed to the continued loss of axons in the existing plaques, to the increasing number of lesions, and to generalized brain atrophy. Over 30 years, about half of MS patients will develop sufficient ataxia or spasticity to require a wheelchair and the average life expectancy is shortened by several years. However, about 20 % of patients have a benign clinical course their entire life.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








