Brain Tumors and Other Space-Occupying Lesions
Adam L. Hartman
Ronald P. Lesser
GENERAL OVERVIEW
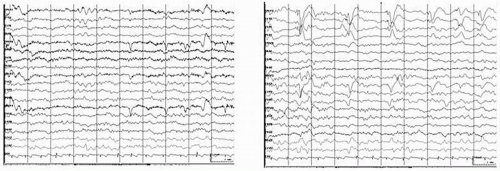
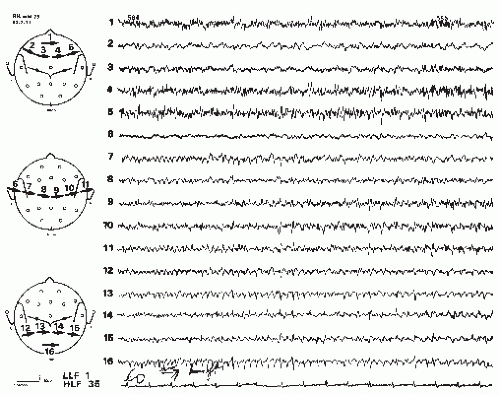
Brain tumors are a leading cause for new-onset seizures in the adults and many patients still present with seizures as their first manifestation of a tumor (Fig. 17.1). Although EEG at one time was important for diagnosing brain tumors and other space-occupying lesions, this is now more commonly done using imaging studies, such as computed tomography (CT) and magnetic resonance imaging (MRI) (Fig. 17.2). Clinical neurophysiology still is important in managing these patients, including the use of electrocorticography (ECoG) during testing to identify eloquent cortex during resection surgeries, application of evoked potentials in assessing the location of sensorimotor cortex or the extent of tumor involvement, and the application of magnetoencephalography, for both magnetic source imaging (e.g., in localizing spike-generating zones) and functional mapping. These topics will be discussed in this chapter.
The EEG findings discussed in this chapter are not specific for tumor type and also can occur with other space-occupying lesions (hence, their inclusion in this chapter). In a series of classical experiments Gloor et al. (1) found that focal delta-range slowing was seen over well-circumscribed lesions in the white matter (with no apparent change in faster frequency activity) or after a focal thalamic lesion, while diffuse hemispheric slowing was seen after larger thalamic and/or hypothalamic lesions or lesions causing cerebral edema; generalized slowing was seen after lesions were made in the midbrain tegmental area. Solid tumors generally are not epileptogenic in themselves; it is the infiltrated or surrounding nontumor tissue that causes the seizures (2,3). Notable exceptions include developmental tumors such as hypothalamic hamartomas (HH; discussed in the section “Hypothalamic Hamartoma”). EEG findings in most nondevelopmental tumors likely are caused by displacement of normal tissue (either by the mass itself or by altered flow of cerebrospinal fluid), physical disruption of normal neuronal circuitry, ischemia, or hemosiderin deposition (4,5), gap-junction proteins (6), and alterations in neurotransmitter pathophysiology, such as vesicular glutamate transporters (7), both ionotropic and metabotropic glutamate receptor subunits (8), and GABA receptors (9). Data on patients with brain tumors provide some of the most convincing arguments for secondary epileptogenesis (wherein an epileptogenic region gives rise to a secondary epileptogenic focus via strong connectivity between the two regions) (10).
Epidemiology
The prevalence of seizures depends in part on type of tumor: for example, anywhere from 66% to 90% of patients with low-grade astrocytomas have seizures at some point (11).
Seizures are more common in patients with low-grade (vs. high-grade) tumors (12). They are the presenting symptom in up to 76% of children with benign astrocytic and oligodendrocytic tumors of the cerebral hemispheres (13). Seizures occurred in 40% of children with hemispheric brain tumors (with the temporal lobe being most commonly involved), while none with thalamic tumors had seizures (14). There was a focal component to the majority of the seizures in that series, although nearly 25% of the patients had only generalized seizures. Seizures can occur in children with supratentorial or infratentorial tumors, although they are more commonly the presenting sign in hemispheric tumors (15). Conversely, only up to 11% of patients with a “possible seizure” have brain tumors diagnosed (16).
Seizures are more common in patients with low-grade (vs. high-grade) tumors (12). They are the presenting symptom in up to 76% of children with benign astrocytic and oligodendrocytic tumors of the cerebral hemispheres (13). Seizures occurred in 40% of children with hemispheric brain tumors (with the temporal lobe being most commonly involved), while none with thalamic tumors had seizures (14). There was a focal component to the majority of the seizures in that series, although nearly 25% of the patients had only generalized seizures. Seizures can occur in children with supratentorial or infratentorial tumors, although they are more commonly the presenting sign in hemispheric tumors (15). Conversely, only up to 11% of patients with a “possible seizure” have brain tumors diagnosed (16).
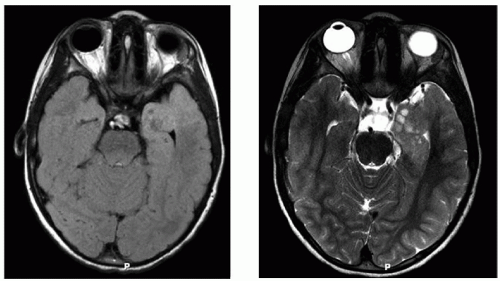 Figure 17.2 MRI of the patient from Figure 17.1. Axial sections (FLAIR and T2-weighted sequences) at the level of the temporal lobes show cystic changes, swelling, and infiltration of normal brain parenchyma in the left anterior mesial temporal lobe. The patient had an MRI soon after his clinic appointment, underwent a near-total resection of a WHO grade II astrocytoma, and has remained seizure-free since surgery. |
Historical Perspectives on the Utility of EEG
Before the advent of modern imaging, EEG was a critical component in the evaluation of patients with suspected masses, along with pneumoencephalography, ventriculography, and angiography. In fact, serial EEGs were somewhat useful in distinguishing strokes (where EEG abnormalities resolved over time) from tumors (where EEG abnormalities became progressively worse) (4). Postoperative EEGs were used to monitor tumor recurrence, which was heralded by focal arrhythmic slow activity at the former tumor site; a recent study found that EEG slowing indicated that postoperative seizure control was less likely (17). Much of the literature on EEG in tumors and other space-occupying lesions is older and studies were performed without the modern standards of statistical rigor. The classification of tumors also has evolved over time, so we attempted to be as nonspecific as possible in describing tumor type in older studies, while using current tumor nomenclature for more recent studies. One final caveat is that many observers have noted that the routine EEG can be normal or even misleading in terms of lateralization (14,15,18,19), thus limiting the utility of this test in some instances. In fact, normal frequency activity may be seen ipsilateral to a tumor (18).
LOCALIZATION-RELATED ISSUES
Hemispheric Tumors
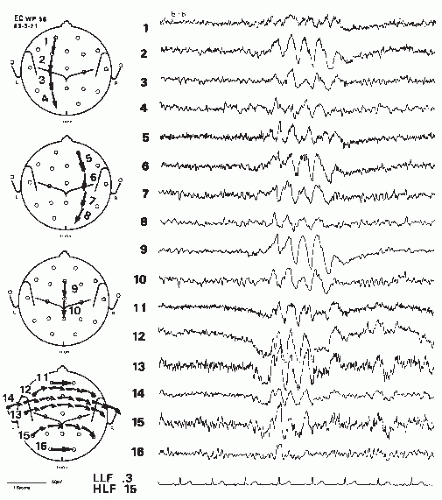
Cortical tumors produce abnormal EEGs >96% of the time, including focal delta activity (correctly localized) (Figs. 17.1 and 17.2); a minority causes focal dysrhythmia, but EEGs can be falsely localizing (Figs. 17.3 and 17.4) (20). EEGs are abnormal in up to 87% of children with benign astrocytic and oligodendrocytic tumors of the cerebral hemispheres (13). EEG findings are generally related to location of the tumor and rapidity of growth. Most (4,21), but not all (22), studies associate rapidly growing tumors with very slow delta-range activity, and more slowly growing tumors with arrhythmic theta-range activity with occasional intermixed epileptiform spikes and sharp waves. It should be noted that up to one third of patients with brain tumors have an epileptogenic focus remote from the tumor site, which may give rise to some confusion in localization (10). Meningiomas and other slow-growing or midline tumors may be difficult to localize, although hyperventilation may reveal a focus of slowing not seen otherwise (20,23). Focal theta-range activity can occur with any type of tumor and one series noted it in 75% of patients with hemispheric tumors (18). In that study, focal delta-range activity was most common in patients with glioblastoma multiforme, although it also occurred with meningeal tumors. Activity in the delta range was less frequent with astrocytomas, where focal sharp activity was more common. Focal epileptiform activity on the EEG appeared to be correlated generally with clinical seizures in patients with astrocytomas, but clinical seizures were not as common among patients with meningeal tumors, even though they also had a fair amount of focal epileptiform activity on EEG, seizures were not as common. Newmark et al. found that epileptiform activity was unrelated to focal slowing in patients with gliomas (22). Patients with multiple metastases also may have a good deal of epileptiform activity in their EEGs; over 90% of patients with metastatic disease have abnormal EEGs including epileptiform activity (4), delta-range activity, and dysrhythmias (20).
Recording from the cortical surface shows similar findings. While focal delta-range activity and/or background attenuation may be seen with some hemispheric tumors, focal slowing can be seen from an area literally centimeters away from the actual tumor (24). Those authors suggested that one potentially helpful localizing sign is resistance to afterdischarge occurrence, even at high current intensities, during cortical stimulation.
Focal delta- and theta-range activity correlates with white matter involvement of tumors (1,22). This includes tumors involving both gray and white matter as well as those involving white matter only. Rhythmic delta activity may indicate involvement of the thalamus, although involvement of deep frontal white matter may produce similar findings (22).
Attenuation of background activity is seen in patients with high-grade gliomas that have thalamic involvement (22) but also may be seen in regions with extensive peritumoral edema (25), although this is a nonspecific finding. Irregular delta-range activity is unrelated to peritumoral edema (22) and the degree of EEG abnormality does not appear to correlate with degree of edema (25).
Tumor was present in 18% of series of 282 patients showing typical periodic lateralized epileptiform discharges (PLEDs) (26).
Frontal tumors tend to produce the slow patterns noted previously, at times showing frontal intermittent rhythmic discharges (FIRDA). Parietal and occipital tumors may affect the posterior basic rhythm, but also may show abnormalities in more anterior regions, possibly leading to false localization (Fig. 17.4). Temporal tumors can show a unilateral abnormality up to 89% of the time (Fig. 17.1) (27). Focal delta activity or sharp waves occurred in half of these patients. In over 90% of temporal gliomas, slowing was continuous (27). A more recent series (which included prolonged video-EEG monitoring) noted temporal epileptiform discharges and focal temporal slowing as the most common finding with temporal lobe tumors, although a few patients also showed bilateral, generalized, or false localization (28).
Posterior Fossa and “Deep” Tumors
Because most tumors in children over 1 year of age are infratentorial, it is important to consider posterior fossa and so-called “deep” (e.g., sellar, chiasmal, and diencephalic) tumors separately. Although most of these patients have had an imaging study, the presenting symptoms can be so nonspecific (e.g., altered mental status) that an EEG might be obtained for other reasons in the course of a patient’s treatment (e.g., to rule out nonconvulsive status epilepticus). Therefore, it is important to understand EEG findings in this context so that an appropriate interpretation of EEG patterns can be made. One found that over three fourth of records was abnormal in children with posterior fossa tumors, with likelihood of abnormality decreasing with age (from below 10 to over 50 years) (23). However, two other studies found EEGs to be normal in 19% (15) and 24% (19) of children. Location within the posterior fossa may be important. EEGs were abnormal in only 30% of patients with brainstem tumors, while over 80% of EEGs were abnormal in those with cerebellar or fourth ventricle tumors (19). In that series, 27% had posterior rhythmic delta waves, 32% had generalized bilateral bursts of rhythmic slowing, 51% had posterior arrhythmic delta waves, and 11% had rhythmic theta or delta waves on vertex or anterior quadrants.
Both Bickford and Martinius et al. noted the suppression of abnormal rhythmic delta-range activity on arousal in patients with deep tumors (19,20). Because these patterns may be produced by tumors distant from where the abnormal EEG patterns are recorded, they are sometimes referred to as “projected” rhythms. EEG may not lateralize, or may falsely lateralize, posterior fossa tumors (19). EEG abnormalities (posterior slow activity) were more common in patients with posterior fossa tumors and evidence of increased intracranial pressure (i.e., evidence of third ventricle dilation) (19). Arrhythmic delta activity is more common in patients with more rapid progression of symptoms, possibly due to rapid expansion of tumor size (19). Nearly half the EEGs of patients with tumors of the cerebellopontine angle (CPA) were normal in one series (23).
Sellar Tumors
EEG abnormalities in tumors of the sellar region include temporal lobe abnormalities, unilateral delta-range activity, and bitemporal dysrhythmia. It is important to remember this in the differential diagnosis of temporal lobe abnormalities. In tumors that compressed the third ventricle, generalized slowing was noted, and degree of compression was the only factor that correlated with abnormalities in the EEG (29). In that series, EEG abnormalities did not predict tumor type (29).
Hypothalamic Hamartoma
HH is a developmental malformation that frequently presents with seizures, including infantile spasms. Other associated conditions may include precocious puberty and behavioral problems. An excellent recent review of the topic is available (30). In children, gelastic seizures are most typical at onset, although this may not be the case in adults, who tend to present with partial-onset seizures (31). The other main cause of gelastic epilepsy is temporal or frontal lobe epilepsy (32). The EEG in HH initially may be normal, similar to other deep-seated masses, although over time it may evolve through the appearance of focal (partial onset), and then generalized seizures, consistent with the clinical semiology (30). There may be associated autonomic features (33). Focal slowing or epileptiform activity over frontal and/or temporal head regions may be the initial appearance on EEG, although this evolves into bilateral spike wave over time if untreated (30). Eventually, a pattern consisting of generalized slow spike wave, paroxysmal fast, and electrodecremental patterns can occur, reminiscent of the EEG in Lennox Gastaut syndrome (34). There is a possibility that the hamartoma in some way generates abnormal activity that propagates through the cortex, leading to the various seizure types noted in HH (30,35). In four patients with HH presenting epileptic manifestations and displaying interictal spikes over the frontal and temporal areas, EEG source analysis, based on scalp recordings (32 electrodes), was able to estimate that the epileptiform spikes have deep sources in the neighborhood of the hamartoma, with later spread to cortical areas (36). A subsequent study from the same group of a patient with HH and gelastic epilepsy using simultaneous EEG and fMRI recordings of several seizures indicated that the epileptic activity appeared to originate in the area around the tumor and propagate through the left fornix to the temporal lobe, and later through the cingulate fasciculus to the left frontal lobe (37).
ELECTROPHYSIOLOGY IN SURGICAL NEURO-ONCOLOGY
Electrophysiology retains a vital role in the surgical management of tumors and other space-occupying lesions. One of the most challenging surgical decisions is balancing the desire to resect as much tumor as possible with the desire to leave intact tissues important to motor, sensory, or language-related functions. Certain masses, particularly tumors with a tendency to infiltrate otherwise normal tissue (e.g., glioblastoma multiforme), pose a real dilemma for the surgeon and oncology team, requiring decisions that may leave the patient either with more residual tumor or without functions critical to optimal daily living. Neurophysiology can offer guidance in defining two important regions: the peritumoral epileptogenic zone and functional cortex (i.e., areas that typically are critical to motor, sensory, or language function and whose resection would lead to significant deficits). Simple lesionectomies also are appropriate in the setting of certain well-defined lesions (e.g., caver-nomas) or those near noneloquent cortex. Data from some series (38,39) suggest lesionectomies may be adequate in some cases, although selection of candidates for this approach has not been thoroughly studied; success in these cases suggests that disruption of an epileptogenic network may suffice to suppress seizure activity (40).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree