Brainstem
In rostrocaudal order, the brainstem consists of three subdivisions, the midbrain, pons, and medulla oblongata. Extending the entire length of the brainstem, any cross section demonstrates three laminae: the tectum, tegmentum, and basis [37].
Medulla Oblongata
Anatomy of the Medulla
The medulla oblongata or myelencephalon is the most caudal portion of the brainstem (Fig. 15.1) and extends from the caudal border of the pons to a point just rostral to the point of emergence of the first spinal nerve roots. The junction of the medulla oblongata and spinal cord is at the level of the foramen magnum. The cross-sectional anatomy at a midmedullary level is illustrated in Figure 15.2.
Within the substance of the medulla certain cranial nerve nuclei and roots are situated [22]. The hypoglossal nucleus (cranial nerve XII) is located near the ventrolateral portion of the central canal under an eminence called the hypoglossal trigone. The nerve roots of the hypoglossal nerve pass ventrally and emerge from the medulla in the anterior lateral sulcus between the pyramids and the olive (inferior olivary prominence). The nucleus ambiguus (cranial nerves IX, X, and bulbar XI) is located within the medullary reticular formation ventromedial to the nucleus and spinal tract of the trigeminal nerve (cranial nerves V, VII, IX, and X). The dorsal motor nucleus of the vagus (cranial nerve X) lies dorsolateral to the hypoglossal nucleus and sends fibers that join the motor roots of the vagus and spinal accessory nerves. The nucleus and tractus solitarius (cranial nerves VII, IX, and X) lie ventrolateral to the dorsal motor nucleus of the vagus, and the medial and spinal vestibular nuclei and the dorsal and ventral cochlear nuclei (cranial nerve VIII) are located at the dorsal and ventral borders of the inferior cerebellar peduncle (restiform body). The inferior olivary nucleus is located within the olive.
The nucleus gracilis and nucleus cuneatus are located in the posterior funiculi of the dorsal medulla and give rise to fibers (internal arcuate fibers) that cross in the decussation of the lemniscus (great sensory decussation). These fibers then travel in the medial lemniscus, which is dorsomedial to the pyramids. The nucleus of the spinal tract of the trigeminal nerve (pars caudalis) lies lateral to the internal arcuate fibers and descends caudally to the level of C3 in the cervical spinal cord, whereas the spinal tract of the trigeminal nerve lies lateral to the nucleus. The pyramids are located in the anterior (ventral) medulla and contain descending corticospinal tract fibers to the lateral and anterior corticospinal tracts of the spinal cord. The pyramid also contains descending corticobulbar fibers. In the caudal end of the medulla, nearly 75% to 90% of the corticospinal fibers in the pyramid cross the ventral midline (decussation of the pyramids or great motor decussation) to the opposite side to form the lateral corticospinal tract. The rest of the corticospinal tract descends homolaterally to form the anterior corticospinal tract. There is a somatotopic organization of the corticospinal fibers within the pyramids, with the fibers of the lower extremities placed more laterally than the fibers of the upper extremities [1]. The medial longitudinal fasciculus is located in the dorsomedial medulla. Other medullary tracts include the ventral and dorsal spinocerebellar tracts, the medial and lateral reticulospinal tracts, the medial and lateral vestibulospinal tracts, the rubrospinal tracts, the spinothalamic tracts, and descending sympathetic pathways.
Vascular Supply of the Medulla
The large regional arteries of the brainstem have the following three types of branches:
1. The paramedian arteries, which penetrate the ventral brainstem surface and supply the midline structures.
2. The short circumferential arteries, which traverse laterally on the brainstem and penetrate its ventrolateral and lateral surfaces.
3. The long circumferential arteries, which course around the brainstem and supply its posterior structures and cerebellum.
The medulla oblongata receives its blood supply from the anterior and posterior spinal arteries, the posterior inferior cerebellar artery, and branches of the vertebral arteries. The blood supply to the medulla may be subdivided into two groups: the paramedian bulbar branches and the lateral bulbar branches.
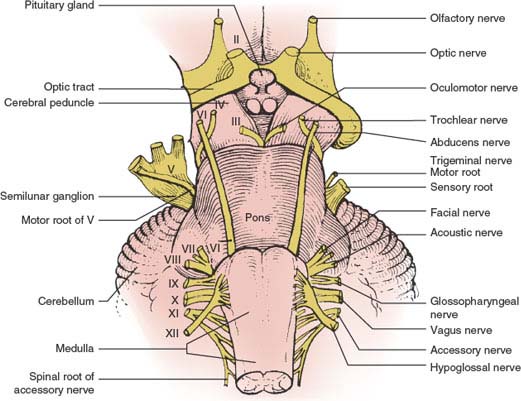
FIG. 15.1. The brainstem (ventral view).
PARAMEDIAN BULBAR BRANCHES
The paramedian portion of the medulla (the hypoglossal nucleus and emergent nerve fibers, the medial longitudinal fasciculus, the medial lemniscus, the pyramids, and the medial part of the inferior olivary nucleus) are supplied by the vertebral artery. At lower medullary levels, the anterior spinal artery also contributes to the paramedian zone.
LATERAL BULBAR BRANCHES
The lateral portion of the medulla is supplied by the intracranial vertebral artery (fourth segment) or the posterior inferior cerebellar artery. Occasionally, the basilar artery or the anterior inferior cerebellar artery also contributes.
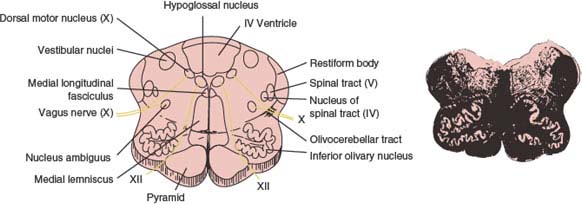
FIG. 15.2. Midportion of the medulla at the origin of the hypoglossal and vagus nerves. Myelin-stained section is shown at right. (From Daube JR, Reagan TJ, Sandok BA, et al. Medical neurosciences: an approach to anatomy, pathology, and physiology by system and levels, 2nd ed. Boston, MA: Little, Brown and Company, 1986. By permission of Mayo Foundation.)
Medullary Syndromes
MEDIAL MEDULLARY SYNDROME (DEJERINE’S ANTERIOR BULBAR SYNDROME)
This syndrome often results from atherosclerotic occlusion of the vertebral artery, anterior spinal artery, or the lower segment of the basilar artery. Vertebrobasilar dissection, dolichoectasia of the vertebrobasilar system, embolism, and meningovascular syphilis are less common causes of the medial medullary infarction [155]. The anterior spinal artery supplies the paramedian region of the medulla oblongata, which includes the ipsilateral pyramid, medial lemniscus, and hypoglossal nerve and nucleus (Fig. 15.3). Its occlusion therefore results in the following signs:
1. Ipsilateral paresis, atrophy, and fibrillation of the tongue (due to cranial nerve XII affection). The protruded tongue deviates toward the lesion (away from the hemiplegia). Cranial nerve XII function may be spared [127].
2. Contralateral hemiplegia (due to involvement of the pyramid) with sparing of the face.
3. Contralateral loss of position and vibratory sensation (due to involvement of the medial lemniscus). The more the dorsolateral spinothalamic tract is unaffected, the more the pain and temperature sensation are spared.
4. Occasionally, upbeat nystagmus may occur because of dorsal extension of the infarct toward the medial longitudinal fasciculus [70]. Unilateral lesions of the nucleus intercalatus can account for primary position upbeat nystagmus due to a unilateral medial medullary infarction [69]. It has also been proposed that damage to the uncrossed climbing fibers from the inferior olivary nucleus to the contralateral cerebellar Purkinje cells results in ocular contrapulsion from rostral medial medullary infarctions [81].
The medial medullary syndrome may occur bilaterally [57,99] resulting in flaccid quadriplegia (with facial sparing), bilateral lower motor neuron lesions of the tongue, complete loss of position and vibratory sensation affecting all four extremities and respiratory failure, or acute onset of triparesis (with involvement of both lower limbs and contralateral upper extremity), suggestive of a possible fiber segregation of the descending tracts of different extremities [58]. Located in the caudal medullary tegmentum, both hypoglossal nuclei have been involved in isolation in a small medullary infarction [12].
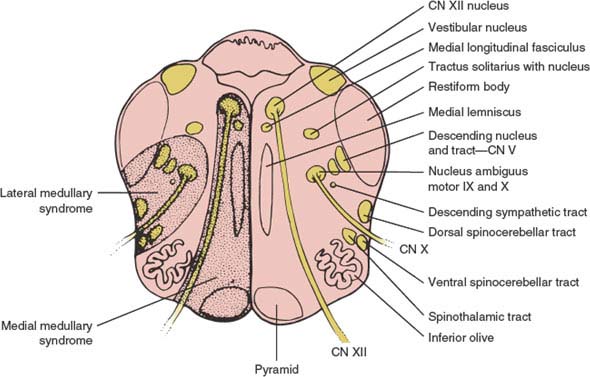
FIG. 15.3. Cross section of medulla oblongata showing area involved in medial medullary infarction and lateral medullary infarction (Wallenberg syndrome). CN = cranial nerve.
Because the hypoglossal fibers run somewhat laterally to the medial lemniscus and pyramid, they are occasionally spared in cases of anterior spinal artery occlusion. Occasionally, only the pyramid is damaged, resulting in a pure motor hemiplegia that spares the face [30,128,135]. Central facial paresis may also result from a unilateral contralateral medullary infarction, suggesting that some of the facial corticobulbar fibers descend ipsilaterally before making a loop as low as the medulla oblongata before decussating and ascending to the contralateral facial nucleus that innervates the perioral musculature [26,148]. A crossed motor hemiparesis (hemiplegia cruciata), with paralysis of the ipsilateral arm and the contralateral leg (resulting from a lower medullary lesion compromising the crossed fibers to the arm as well as the uncrossed fibers to the leg), is an extremely rare occurrence [14].
Apart from incomplete syndromes (e.g., medial medullary syndrome presenting as pure motor hemiparesis, or medial medullary syndrome without tongue paralysis), other unusual neurologic findings may be observed including contralateral paralysis of the pharyngeal constrictor muscle [111] and contralateral tongue paralysis [27].
LATERAL MEDULLARY (WALLENBERG) SYNDROME
This syndrome [33,34,54,80,107,114,132] is most often secondary to intracranial vertebral artery or posterior inferior cerebellar artery occlusion [78]. The presumed pathogenesis among 130 consecutive patients with pure lateral medullary infarctions included large vessel disease in 50%, arterial dissection in 15%, small vessel disease in 13%, and cardioembolism in 5% [78]. Spontaneous dissections of the vertebral arteries are a common cause [75,105]. Dissections were observed more often with caudal lesions [78]. The syndrome has also been described with cocaine abuse [104], medullary neoplasms (usually metastases), abscess, demyelinating disease [141], radionecrosis, hematoma (secondary to rupture of a vascular malformation), neck manipulation [56], trauma, bullet injury to the vertebral artery [102], and posterior spinal fusion surgery with instrumentation in a patient with a previously undiagnosed Chiari 1 malformation [122]. The characteristic clinical picture results from damage to a wedge-shaped area of the lateral medulla (Fig. 15.3) and inferior cerebellum and consists of several signs:
1. Ipsilateral facial hypalgesia and thermoanesthesia (due to trigeminal spinal nucleus and tract involvement). Ipsilateral facial pain is common [34].
2. Contralateral trunk and extremity hypalgesia and thermoanesthesia (due to damage to the spinothalamic tract).
3. Ipsilateral palatal, pharyngeal, and vocal cord paralysis with dysphagia and dysarthria (due to involvement of the nucleus ambiguus).
4. Ipsilateral Horner syndrome (due to affection of the descending sympathetic fibers). Ipsilateral hypohidrosis of the body may occur, probably due to interruption of the mostly uncrossed excitatory sweating pathway, which descends from the hypothalamus through the tegmental area of the mesencephalon and pons and, more caudally, through the posterolateral area of the medulla to synapse with the sympathetic sudomotor neurons of the intermediolateral cell column of the spinal cord [84].
5. Vertigo, nausea, and vomiting (due to involvement of the vestibular nuclei).
6. Ipsilateral cerebellar signs and symptoms (due to involvement of the inferior cerebellar peduncle and cerebellum).
7. Occasionally, hiccups (singultus) attributed to lesions of the dorsolateral region of the middle medulla [117] and diplopia (perhaps secondary to involvement of the lower pons).
Lateral lesions located in the rostral medulla are associated with more severe dysphagia, hoarseness, and the presence of facial paresis, whereas more caudal lesions situated in the lateral surface of the medulla, correlate with more marked vertigo, nystagmus, and gait ataxia [80]. Nausea, vomiting, and Horner syndrome are common regardless of the location of the lesion in the lateral medulla; lesions that extend more ventromedially cause facial sensory changes on the contralateral side of the lesion [80]. The motor system (pyramids), tongue movements, and vibration and position sense are typically spared with lateral medullary lesions because the corresponding anatomic structures are located in the medial medulla. The triad of Horner syndrome, ipsilateral ataxia, and contralateral hypalgesia clinically identifies patients with lateral medullary infarction [132]. Cerebellar infarcts only infrequently accompany the lateral medullary syndrome, suggesting that most of the posterior inferior cerebellar artery territory is spared, despite the high frequency of vertebral artery occlusion as a cause of this syndrome [132].
Headache, especially unilateral headache localized to the upper posterior cervical region, is relatively common with the lateral medullary syndrome, particularly when the syndrome is due to cervical vertebral artery dissection [64,105]. This type of headache should be distinguished from the rare paroxysmal retro-orbital hemicranial-like attacks reported after strokes involving the dorsal medulla and high cervical spinal cord at the C1 level [36].
The sensory defect in the lateral medullary syndrome usually affects the ipsilateral face and the contralateral leg, arm, and trunk. However, several patients with lateral brainstem lesions developed a sensory defect involving the ipsilateral face and the contralateral foot, with the latter defect extending upward to end in a sensory level [96]. These patients with a crossed pattern of sensory defect had far lateral lesions of the lateral medulla and pons, with the leg and lower torso involvement due to selective partial disruption of the somatotopically organized sacral and lumbar afferent fibers of the lateral spinothalamic tract (located far laterally in the brainstem), with sparing of the more medial thoracic and cervical fibers [96]. Several patients have also been described with a continuous hemisensory defect of the face, arm, and trunk (unilateral pattern), with the lower border demarcated at a sensory level [96]. These patients were thought to have mediolateral medullary and pontine lesions contralateral to the side of the sensory defect, which affected the medial cervical and thoracic afferents of the lateral spinothalamic tract (i.e., spared the lateral sacral and lumbar afferents) and the ventral trigeminothalamic tract (accounting for contralateral facial sensory loss), but spared the spinal nucleus and tract of the trigeminal nerve. In rare instances of infarcts involving the pontomedullary sulcus, sensory symptoms electively involve the contralateral upper limb and base of the neck resulting in loss of pain and temperature, and reinforcing the notion that a somatotopic arrangement of the spinothalamic tract in its medullary course [160].
Rare manifestations of the Wallenberg syndrome include the following:
1. Wild arm ataxia probably related to involvement of the lateral cuneate nucleus [32,33].
2. Clumsiness of the ipsilateral upper limb resulting from extension of the injury into the subolivary area [22].
3. Central pain associated with allodynia [121].
4. Contralateral hyperhidrosis with ipsilateral anhidrosis due to interruption of the sympathetic pathways (noted a few months after infarction) [130].
5. An inability to sneeze due to compromise of the sneezing center located at the ventromedial margin of the descending tract and nucleus (spinal nucleus) of the trigeminal nerve [68].
6. Paroxysmal sneezing due to presumed involvement of the hypothetical human “sneezing center” in the rostral dorsolateral medulla [45,113,137].
7. Loss of taste that results from involvement of the rostral and the lateral zone of the nucleus tractus solitarius [59].
8. Autonomic dysfunction including tachycardia, blood pressure lability, and respiratory failure from the involvement of the caudal and medial zone of the nucleus tractus solitarius [25].
9. Failure of automatic breathing (Ondine’s curse) due to discrete lesions of the nucleus ambiguus and the adjacent reticular formation.
10. Transient urinary retention from interruption of descending fibers from facilitatory pontine micturition centers [89].
11. Body lateropulsion without limb ataxia from the involvement of the descending lateral vestibulospinal tract, or body lateropulsion with limb ataxia due to interruption of the ascending dorsal spinocerebellar tract [151].
12. Axial lateral pulsion that results from the involvement of the vestibulospinal and spinocerebellar tracts as well as central vestibular pathways [7].
13. Isolated ipsiversive lateropulsion [3].
14. Pure sensory stroke with loss of pain and temperature involving the face, arm, trunk, and leg as the only manifestations of the lateral medullary infarction [8,15].
15. Ipsilateral sensory symptoms predominantly involving the upper extremities, especially the fingers, with occasional impairment of vibration and position sense from caudal lesions involving the dorsal columns or decussating lemniscal fibers [79].
16. Ipsilateral hemiparesis from the involvement of the lower most caudal end of the medulla just below the pyramidal decussation [38]. An ipsilateral spastic hemiplegia associated with a lateral medullary syndrome is also known as the submedullary syndrome of Opalski (see subsequent text) [106].
17. Central hypoventilation is seen along with vasomotor instability [87].
18. Poststroke facial pain that results from the involvement of the primary afferent fibers in the descending spinal trigeminal tract [53].
Various abnormalities of eye movements and vision have been described with the lateral medullary syndrome (Table 15.1) [18,21,29,39,100]. These include the following:
1. Dysfunction of ocular alignment. Lateral medullary lesions damage the otolithic vestibular nuclei and, therefore, patients with Wallenberg syndrome often demonstrate skew deviation with hypotropia on the side of the lesion [77]. Brandt and Dieterich have called this type 2 skew deviation and stated that this skew results from elevation of the contralateral eye, without vertical displacement of the ipsilateral eye [19,20]. Some patients also show an ipsilateral head tilt and a disconjugate ocular torsion (the ocular tilt reaction, see Chapter 8) with excyclodeviation of the ipsilateral lower eye but with little or no incyclodeviation of the contralateral higher eye [20,39,107]. Therefore, patients may complain of diplopia with images displaced vertically and tilted with respect to each other. Some patients with Wallenberg syndrome may also exhibit ocular ipsipulsion due to damage to the climbing fibers from the contralateral inferior olivary nucleus to the dorsal vermis [82] or complain of the unusual (and almost unbelievable) sensation of environmental tilt, in which the whole room is tilted on its side or even upside down (“floor-on-ceiling” phenomenon) [39,127]. This syndrome is also probably caused by a disturbance of vestibular-otolith central connections [127]. Environmental tilt or “upside down” reversal of vision may also occur with vertebrobasilar transient ischemic attacks [143], vertebrobasilar ischemia [144], encephalitis, head injury [100], demyelinating disease [138], or after third ventriculostomy for hydrocephalus [116].
TABLE 15.1 Ocular Motor Abnormalities in Wallenberg Lateral Medullary Syndrome

Damage to otolithic central projections mediating ocular counter-roll may also contribute to the genesis of torsional nystagmus (see subsequent text) in the lateral medullary syndrome [107]. Central otolithic involvement may also be responsible for the see-saw nystagmus observed in occasional patients [63,103]. See-saw nystagmus is a disjunctive, vertical-torsional nystagmus half cycle, which consists of elevation and intorsion of one eye with synchronous depression and extorsion of the other eye; the next half cycle consists of the reversal of these vertical and torsional movements. This type of nystagmus is usually pendular and noted especially with large, extensive suprasellar lesions that compress or infiltrate the mesodiencephalon bilaterally. With lateral medullary lesions, however, a jerk see-saw nystagmus may occur [63,107]. The torsional component of this nystagmus is conjugate with the fast component contraversive to the side of the lesion [63]. This contrasts with the jerk see-saw nystagmus described with unilateral, focal mesodiencephalic lesions, in which the quick phase of the torsional component is toward the side of the lesion [63].
2. Nystagmus. Nystagmus in the lateral medullary syndrome may be due to direct damage to the vestibular nuclei or their cerebellar, semicircular canal, or otolithic connections. Nystagmus in the lateral medullary syndrome is usually positional and can be horizontal [42], torsional [107], or mixed, with torsion, vertical, and horizontal components [10]. Typically, horizontal nystagmus beats away from the side of the lesion, with the horizontal drift velocity directed toward the side of the lesion being influenced by eye position and by fixation. Occasionally, the nystagmus may beat with the fast component ipsilaterally during gaze toward the side of the lesion or during eye closure [10]. A vertical nystagmus is usually upbeating [10]. The nystagmus is often evident only in the initial days after dorsolateral medullary infarction, and rapidly declines over the following days [125]. Torsional nystagmus is common with Wallenberg syndrome, with the upper pole of the iris beating away from the side of infarction [107]. Torsional nystagmus has been attributed to an imbalance of central projections from the anterior and posterior semicircular canals and the otolithic receptors that mediate ocular counter-roll [107].
As mentioned in the preceding text, see-saw nystagmus may also occur with lateral medullary lesions [103]. Gaze-evoked eyelid nystagmus associated with ocular nystagmus has been described, in which a clinically obvious upward jerking of the lids occurred synchronously with the fast phase of a gaze-evoked horizontal nystagmus [35]. This eyelid nystagmus was inhibited or totally arrested by the near reflex.
3. Smooth pursuit and gaze-holding abnormalities. Structures and pathways located in the lateral medulla are also concerned with smooth pursuit eye movements and gaze holding [162]. The cerebellar flocculus, paraflocculus, and vermis climbing fibers pass through the inferior cerebellar peduncle and are concerned with these functions.
Patients with the lateral medullary syndrome may complain of a sensation of their bodies being pulled to one side and attempt to counteract this lateropulsion of the body by leaning toward the opposite side. Because of gaze-holding impairment, ocular movements may be similarly affected, with a tendency for the eyes to be “pulled” toward the involved medulla (lateropulsion or ipsipulsion of eye movements) [10,42,55,98,157,161]. If a patient is asked to fixate straight ahead and close the eyelids, the eyes will deviate toward the side of the medullary lesion (reflected by a series of small corrective hypometric saccadic [fast] eye movements in the opposite direction, which are directed to fixation when the eyes are again opened). Even blinking may induce this lateropulsion. These abnormalities of gaze holding may also be reflected in saccadic eye movement abnormalities. Smooth pursuit eye movements tracking targets moving away from the side of the lesion are also impaired with lateral medullary lesions, whereas pursuit toward the side of the lesion is normal, or nearly so [10,98,162].
4. Abnormalities of saccades. The cerebellum may be involved in modulating the amplitude but not the speed of saccadic (fast) eye movements. Interruption of cerebellar central connections that traverse the lateral medulla probably accounts for some of the observed ocular motor deficits [132]. Damage to the juxtarestiform body, which carries signals from the fastigial nucleus to the brainstem reticular formation, may account for a saccadic abnormality referred to as lateropulsion of saccadic eye movements [90].
As noted in the preceding text, gaze-holding abnormalities in patients with Wallenberg syndrome may result in ipsipulsion of eye movements. This disorder of gaze holding may also induce saccadic abnormalities. Horizontal saccades away from the side of the lesion are hypometric (undershoot the target), whereas saccades directed toward the side of the lesion are hypermetric (overshoot the target) [161]. Quick phases of nystagmus are similarly affected. Ipsipulsion with lateral medullary lesions is therefore opposite to the contrapulsion of saccades that occurs with lesions of the superior cerebellar peduncle [126,157].
Patients with Wallenberg syndrome may have permanent saccadic dysmetria (hypermetria to the side of the lesion and hypometria to targets contralateral to the lesion) and a reduced capability to readjust saccadic amplitude [161]. This horizontal saccade bias with lateral medullary lesions is also reflected in vertical eye movements. On attempting to make a purely vertical saccade, an oblique or elliptical saccade directed toward the lesion (in the direction of lateropulsion) is made, requiring corrective saccades away from the side of the lesion to bring the eyes back toward the intended target. Later, attempted vertical saccades may take on S-shaped trajectories as an adaptive strategy to correct the saccadic dysmetria [90]. Even a torsional component of this bias may occur (torsipulsion), with inappropriate torsional fast eye movements induced during saccades toward or away from the side of the medullary lesion [107].
The medial branch of the posterior inferior cerebellar artery supplies the dorsolateral medulla; infarcts of this branch may be clinically silent, cause isolated vertigo often misdiagnosed as labyrinthitis, cause vertigo associated with ipsilateral lateropulsion of the trunk and gaze and dysmetria or unsteadiness, or cause a full Wallenberg syndrome [5,6,62,73]. Bilateral cerebellar infarction in the territory of the medial branches of the posterior inferior cerebellar arteries may cause vertigo, dysarthria, dysequilibrium with retropulsion, bilateral gaze-evoked nystagmus, and marked gait ataxia without brainstem signs [145]. Vertigo and upside-down vision have been described because of an infarct in the cerebellar flocculus and nodulus due to affection of the medial branch of the posterior inferior cerebellar artery [28].
Atherosclerotic occlusion or dissection of the intracranial vertebral artery can lead to a total unilateral hemimedullary (Babinski-Nageotte) syndrome, a combination of the medial and lateral medullary syndromes [109]. This rare syndrome is characterized by contralateral hemiplegia and sensory loss of the limbs and trunk, ipsilateral hemiataxia, and facial sensory loss, along with dysphagia, dysphonia, and dysarthria. Ipsilateral hemiparesis is extremely rare [93]. Some authorities have suggested Reinhold’s syndrome as the proper eponym for the hemimedullary syndrome [85]. Because of the separate arterial topography supplying the medulla, the simultaneous occurrence of ischemic lesions involving the lateral and medial parts of the medulla is extremely rare [109]. Combinations of the two major syndromes may also occur as bilateral medial and bilateral lateral medullary syndromes [59].
Tegmental medullary lesions (e.g., glioma) may cause lack of appetite and early satiety (medullary satiety), implying that the medulla may play a role in the regulation of feeding behaviors [94]. Lesions affecting the obex of the medulla may result in neurogenic pulmonary edema [140]. This supports the hypothesis that lesions of caudal brainstem structures, especially the nucleus tractus solitarius, the dorsal motor nucleus of the vagus, and the medial reticular formation are responsible for the generation of neurogenic pulmonary edema. Lesions of the area postrema, an emetic center located in the caudal part of the fourth ventricle and lacking a blood—brain barrier, lesions of the dorsolateral pontine tegmentum, as well as other lesions of the lower brainstem, may account for vomiting, often out of proportion to dizziness [50].
OPALSKI (SUBMEDULLARY) SYNDROME
When ipsilateral hemiplegia is associated with symptoms of a lateral medullary syndrome, it corresponds to the submedullary syndrome of Opalski. Opalski syndrome results from an occlusion of the vertebral artery. The ipsilateral hemiplegia is due to a lesion of the lower medulla involving the corticospinal tract after the pyramidal decussation [71,115].
LATERAL PONTOMEDULLARY SYNDROME
This syndrome [48] may result from occlusion of an aberrant arterial branch arising from the upper vertebral artery and running superiorly and laterally to the region of exit of cranial nerves VII and VIII from the pons. It may also occur with pontine hemorrhage [4]. The clinical findings are those seen in the lateral medullary syndrome plus several pontine findings, which includes the following:
1. Ipsilateral facial weakness (due to involvement of cranial nerve VII)
2. Ipsilateral tinnitus and, occasionally, hearing disturbance (due to involvement of cranial nerve VIII)

FIG. 15.4. Cross section of the lower pons at the level of cranial nerves VI and VII. Myelin-stained section is shown on the right. (From Daube JR, Reagan TJ, Sandok BA, et al. Medical neurosciences: an approach to anatomy, pathology, and physiology by system and levels, 2nd ed. Boston, MA: Little, Brown and Company, 1986. By permission of Mayo Foundation.)









