Cranial Nerve XII (The Hypoglossal Nerve)
Anatomy of Cranial Nerve XII (The Hypoglossal Nerve)
The hypoglossal nerve is the motor nerve of the tongue [5]. Its fibers arise from the hypoglossal nucleus, a longitudinal cell column in the paramedian medulla that lies beneath the hypoglossal trigone of the floor of the fourth ventricle. The column of cells extends from the caudal-most medulla oblongata to the medullary-pontine junction. From the hypoglossal nucleus, the nerve fibers travel in a ventrolateral direction through the medullary reticular formation and medial portion of the inferior olive, coursing immediately lateral to the medial longitudinal fasciculus, medial lemniscus, and pyramid.
The fibers of the hypoglossal nerve emerge from the medulla in the preolivary sulcus between the inferior olivary complex and the pyramid as 10 to 12 rootlets that are located medial to cranial nerves IX (the glossopharyngeal), X (the vagus), and XI (the spinal accessory). These rootlets unite into two bundles that pass separately through the dura mater and the hypoglossal canal of the skull.
After leaving the skull in the hypoglossal canal (anterior condylar foramen), the two nerve bundles unite and descend vertically through the neck to the angle of the mandible. During this course, the hypoglossal nerve is quite near the internal carotid artery and the internal jugular vein. In the neck, the nerve passes toward the hyoid bone and then turns medially toward the tongue. It courses over the internal and external carotid arteries and eventually lies beneath the digastric, stylohyoid, and mylohyoid muscles. The nerve passes between the mylohyoid and hypoglossus muscles and then breaks up into a number of branches (the muscular or lingual branches), which supply the various tongue muscles.
The descending hypoglossal ramus, which courses downward to form the ansa hypoglossi, is given off from the hypoglossal nerve proper in the neck. The ansa hypoglossi is formed by the descending hypoglossal ramus (CN XII and C1 cervical root) and the descending cervical ramus (C2 and C3 cervical roots). (The ansa hypoglossi is discussed further in Chapter 3).
The hypoglossal muscular or lingual branches supply all the intrinsic muscles of the tongue (longitudinal, transverse, and vertical muscles) and also the hyoglossus, styloglossus, genioglossus, and geniohyoid muscles (extrinsic muscles of the tongue).
Supranuclear control of the tongue [5,20] is mediated by corticobulbar fibers that originate mainly within the lower portion of the precentral gyrus (perisylvian) area. The cortical area for controlling tongue movement may be the most lateral part of the precentral gyrus lateral to the precentral knob as a small ischemic lesion, causing supranuclear tongue deviation in one patient, was located lateral to the precentral knob of the precentral gyrus [49]. The corticobulbar fibers controlling the genioglossus muscles are crossed; the other tongue muscles appear to have bilateral supranuclear control. Cortico-hypoglossal fibers branch off the main ventral pyramidal tract [42]. Cortical projections to the hypoglossal nucleus cross the midline in the pontomedullary junction and enter the hypoglossal nucleus from its lateral aspect [42].
Clinical Evaluation of Cranial Nerve XII
The clinical evaluation of cranial nerve XII function consists of observation of the tongue at rest and with protrusion and assessment of the strength and rapidity of tongue movements. Although the hypoglossal nerve does contain some proprioceptive afferents, the nerve is otherwise a purely motor efferent nerve, and thereby nerve lesions do not result in sensory abnormalities.
Unilateral lesions of the hypoglossal nerve result in paresis, atrophy, furrowing, fibrillations, and fasciculations that affect the corresponding half of the tongue. This unilateral paresis is best demonstrated by voluntary tongue protrusion, during which the tongue deviates to the side of paresis, mainly because of the unopposed action of the normal contralateral genioglossus muscle (assisted by the geniohyoid). With unilateral lesions, dysarthria and dysphagia are minimal, but difficulty with manipulating food in the mouth is often evident.
In rare cases of hypoglossal nerve damage, motor denervation induce so-called denervation pseudohypertrophy of the tongue [13]. This features extensive fatty replacement in contrast to true hypertrophy where there is an increase in number or size of muscle fibers.
It may be possible to distinguish between extrinsic and intrinsic tongue muscle weakness in patients with unilateral hypoglossal palsy [32]. In a patient with multiple cranial nerve palsies, including a unilateral hypoglossal nerve palsy, tongue protrusion deviated to the right [32]. When the tongue was not protruded, however, the patient could readily turn the tip of the tongue to the left but not the right. The author noted that protrusion of the tongue requires the action of extrinsic tongue muscles, whereas lateral movements of the nonprotruded tongue are accomplished by intrinsic muscles. Protrusion in the patient was accomplished by the unopposed action of the normal contralateral genioglossus, whereas the tongue tip could not be turned to the side of the lesion due to impairment of contraction of the ipsilateral intrinsic muscles (especially the superior and inferior longitudinal muscles) [32].
Bilateral lower motor neuron lesions of the tongue result in bilateral atrophy, weakness, and fibrillations of the tongue. The tongue therefore cannot be protruded voluntarily. This bilateral affection results in a marked difficulty with articulation, especially with the pronunciation of d and t phonemes. Dysphagia is prominent, and breathing difficulties may occur when the flaccid tongue falls backward to obstruct the pharynx.
Localization of Lesions Affecting Cranial Nerve XII
Supranuclear Lesions
Lesions of the corticobulbar tract anywhere in its course from the lower precentral gyrus to the hypoglossal nuclei may result in tongue paralysis [41]. In a large study of patients with cerebral infarction, the frequency of tongue deviation was 29%, and marked facial/brachial paresis or hemiparesis was usually associated [41]. The cortical area for controlling tongue movement may be the most lateral part of the precentral gyrus lateral to the precentral knob as a small ischemic lesion, causing supranuclear tongue deviation in one patient, was located lateral to the precentral knob of the precentral gyrus [49]. Because supranuclear control of the genioglossus muscle originates mainly from the contralateral cortex, a lesion of the corticobulbar fibers above their decussation may result in weakness of the contralateral half of the tongue. Therefore, with an internal capsular lesion, the tongue may deviate toward the side of the hemiplegia. A supranuclear lesion is not accompanied by atrophy or fibrillations of the tongue.
Interruption of the cortico-lingual pathway to the tongue is crucial in the pathogenesis of dysarthria following strokes affecting the internal capsule, basis pontis, or corona radiata [43]. Sudden isolated dysarthria may occur with lacunar infarcts affecting the contralateral corona radiata or internal capsule, which interrupts in isolation the cortico-lingual pathways to the tongue (central monoparesis of the tongue) [44].
Pontine lesions at the ventral paramedian base close to the midline affect the contralateral cortico-hypoglossal projections, whereas lateral lesions at the pontine base affect ipsilateral projections [42]. Lesions at the paramedian dorsal pontine base do not involve the cortico-hypoglossal projections. Lesions of the dorsolateral and mediolateral medulla impair only ipsilateral cortico-hypoglossal projections [42]. This suggests that the main decussation of supranuclear projections to the hypoglossal nucleus in the brainstem is located close to the pontomedullary junction [42]. However, a patient has been described with contralateral glossoplegia due to a ventromedial lesion of the upper medulla [7]. In this patient with contralateral supranuclear glossoplegia, the lesion was located in the ventromedial part of the rostral medulla. This finding showed that the cortico-hypoglossal projections in this patient decussated at the upper medullary level, more caudally than the pontomedullary junction.
Bilateral upper motor neuron affection of the corticobulbar fibers to the hypoglossal nuclei results in a paretic tongue with no atrophy or signs of denervation. Lateral tongue movements are slow and irregular owing to poor supranuclear control (“spastic tongue”), and a spastic dysarthria is evident.
Nuclear Lesions and Intramedullary Cranial Nerve XII Lesions
Unilateral lesions of the hypoglossal nucleus or nerve result in paresis, atrophy, furrowing, fibrillations, and fasciculations that affect the corresponding half of the tongue. Because of the close proximity of the two hypoglossal nuclei, dorsal medullary lesions (e.g., multiple sclerosis, syringobulbia) often result in bilateral lower motor neuron lesions of the tongue. The nuclei may also be affected in motor neuron disease (amyotrophic lateral sclerosis) and in poliomyelitis (bulbar type). Isolated hypoglossal nerve palsy has been reported in association with infectious mononucleosis, presumably due to viral infection of the hypoglossal nerve nucleus [10]. Isolated total tongue paralysis has been described as a manifestation of the bilateral medullary infarction affecting the two nuclei of cranial nerve XII [3].
The hypoglossal nerve may be injured, usually unilaterally, anywhere along its course in the medulla. Intramedullary hypoglossal involvement is suggested by the associated affection of the medial lemniscus, pyramid, or other neighboring intramedullary structures. Processes affecting the hypoglossal nerve in its intramedullary course include tumor, demyelinating disease, syringobulbia, and vascular insult. A rare but characteristic syndrome that affects the hypoglossal nerve in its intramedullary course is the medial medullary syndrome (Dejerine’s anterior bulbar syndrome). This syndrome results from occlusion of the anterior spinal artery or its parent vertebral artery. The anterior spinal artery supplies the ipsilateral pyramid, medial lemniscus, and hypoglossal nerve; its occlusion therefore results in three main signs:
1. Ipsilateral paresis, atrophy, and fibrillations of the tongue (due to affection of cranial nerve XII). The protruded tongue deviates toward the lesion (away from the hemiplegia).
2. Contralateral hemiplegia (due to involvement of the pyramid) with sparing of the face.
3. Contralateral loss of position and vibratory sensation (due to involvement of the medial lemniscus). Because the more dorsolateral spinothalamic tract is unaffected, pain and temperature sensations are spared.
This medial medullary syndrome may occur bilaterally, resulting in quadriplegia (with facial sparing), bilateral lower motor neuron lesions of the tongue, and a complete loss of position and vibratory sensation affecting all four extremities [23].
Because the hypoglossal fibers run somewhat laterally to the medial lemniscus and pyramid, they are occasionally spared in cases of anterior spinal artery occlusion.
Peripheral Lesions of Cranial Nerve XII
Cranial nerve XII has a close spatial relationship with cranial nerves IX (glossopharyngeal), X (vagus), and XI (spinal accessory) in the posterior cranial fossa and as it leaves the skull in the hypoglossal canal. A basilar skull lesion (e.g., tumor or trauma) [34,46] may involve the twelfth cranial nerve alone, producing an isolated cranial nerve XII lower motor neuron lesion; frequently, the other lower cranial nerves (IX, X, and XI) are variably involved as well. When all four of these nerves are damaged (e.g., by a skull fracture through the hypoglossal canal and jugular foramen), a Collet-Sicard syndrome results, consisting of the following signs:
1. Paralysis of the trapezius and sternocleidomastoid muscles (cranial nerve XI)
2. Paralysis of the vocal cord (cranial nerve X) and pharynx (cranial nerve IX)
3. Hemiparalysis of the tongue (cranial nerve XII)
4. Loss of taste on the posterior third of the tongue (cranial nerve IX)
5. Hemianesthesia of the palate, pharynx, and larynx (cranial nerves IX and X)
Other multiple lower cranial nerve palsy syndromes may occur with lesions in the posterior cranial fossa, in the skull, in the retropharyngeal or retrostyloid space, or in the neck (see Chapter 13). With neck lesions, the cervical sympathetic chain may be involved, resulting in an ipsilateral Horner syndrome (miosis, anhidrosis, and ptosis). Other syndromes include the Collet-Sicard, Villaret’s, Jackson’s, Tapia’s, and Garcin’s syndromes (see Table 13.1). Isolated hypoglossal nerve palsy due to compression by a kinked vertebral artery (hypoglossal-vertebral entrapment syndrome) has been described [1,33]. Skull metastases to the clivus may cause bilateral hypoglossal nerve palsies [30].
Combined abducens nerve and hypoglossal nerve palsies are rare. This is often an ominous combination as may be seen with nasopharyngeal carcinoma (Godtfredsen’s syndrome) and with other clival lesions, especially tumors (three-fourths of which are malignant) [18]. Although the combination of abducens nerve palsy with a hypoglossal nerve palsy usually localizes the pathologic process to the clivus, lower brainstem lesions and subarachnoid processes (e.g., cysticercal meningitis) may also cause this unusual dual cranial nerve impairment [18].
Lesions, usually tumors or chronic inflammatory lesions, of the occipital condyle may cause occipital pain associated with an ipsilateral hypoglossal nerve injury (occipital condyle syndrome) [6,11,25]. These patients complain of continuous, severe, localized, unilateral, occipital pain made worse by neck flexion and often associated with neck stiffness. Rotating the head toward the side of the pain often relieves the discomfort, whereas head rotation to the nonpainful side or suboccipital palpation is unbearable [25]. The pain occasionally radiates anteriorly toward the ipsilateral temporal area or eye. About half of the patients complain of dysarthria, dysphagia, or both, specifically related to difficulty in moving the tongue. On examination, patients hold their neck stiffly and are often tender to palpation over the occipital area on the involved side. The ipsilateral tongue is weak and atrophic and occasionally fasciculations are evident on the involved side. The tongue, when protruded, will deviate to the involved side. Occipital condylar fracture may also cause unilateral or bilateral hypoglossal nerve palsies [21].
The hypoglossal nerve may be injured in isolation in the neck or in its more distal course near the tongue. This results in an ipsilateral lower motor neuron type of paresis of half of the tongue. The causes of this peripheral involvement include carotid aneurysms, aneurysms of a persistent hypoglossal artery, vascular entrapment, spontaneous dissection of the extracranial internal carotid artery, local infections, tuberculosis of the atlantoaxial joint, rheumatoid arthritis, surgical (e.g., carotid endarterectomy) or accidental trauma, birth injuries, neck radiation, epidural abscess of the nasopharyngeal/oropharyngeal carotid space, and tumors of the retroparotid or retropharyngeal spaces, neck, salivary glands, and base of the tongue [2,4,12,15,22,24,27,28,31,35,36,38,39]. Deep cervical lymphadenitis or other infections, including osteomyelitis, meningitis, or viral diseases, may also cause hypoglossal palsies [37]. Unilateral or bilateral hypoglossal neuropathy may occur in patients with hereditary neuropathy with liability to pressure palsy [8,48]. Hemiatrophy of the tongue has been described with Lewis-Sumner syndrome, a peripheral neuropathy characterized by multifocal weakness and wasting and associated sensory impairment, considered to be a variant of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) [45].
In a study of 100 cases of hypoglossal palsy, one-third of the patients showed bilateral involvement [17]. Tumors, predominantly malignant tumors, produced nearly half of the cases, with metastases, chordoma, nasopharyngeal carcinoma, and lymphoma the most common types. Trauma (e.g., gunshot wounds) was the second most common cause. Other etiologies included stroke, Guillain-Barré syndrome, infection, neck surgery, Chiari malformation, multiple sclerosis, and hysteria [17].
After hypoglossal nerve injury from carotid endarterectomy, patients may occasionally develop increasingly severe dysarthria and dysphagia beginning several months after the surgery [47]. Electrophysiologic studies have revealed abnormal coactivation of the genioglossus and styloglossus muscles on the affected side in these patients, suggesting aberrant reinnervation. When aberrant reinnervation occurs, the tongue no longer moves in a coordinated manner, and significant dysarthria ensues.
ABNORMAL TONGUE MOVEMENTS
Various movement disorders may affect the tongue, including drug-induced oral-buccal-lingual dyskinesia, athetosis, palatal myoclonus (rhythmic synchronous tongue movements) [26], and tremor. Choreiform movements of the tongue may result in bizarre lingual movements and an inability to keep the tongue protruded on command (trombone tongue). Galloping tongue refers to an episodic, rhythmic involuntary movement of the tongue that has been described after head and neck trauma, consisting of three waves per second that began as posterior midline focal tongue contractions [16]. These movements lasted approximately 10 seconds in each episode and were not accompanied by other body movements or electroencephalographic abnormalities. They were thought to be of brainstem, perhaps pontine, origin [16]. Episodic tongue movements similar to these have been described in patients with chronic epilepsy, who had isolated posterior wave-like tongue movements that occurred two to three times per second for up to 30 seconds and that coincided with desynchronization of the electroencephalogram (EEG) (thought to be “subcortical seizures”) [14]. Continuous undulating movements of the tongue have been described years after radiation therapy for nasopharyngeal carcinoma [35]. Continuous lingual myoclonus has been described after head injury (EEG normal) and is thought to be a form of branchial myoclonus without palatal myoclonus [40]. Abnormal posturing and movement of the tongue (lingual pseudoathetosis), presumably due to lingual deafferentation, may occur with the neck-tongue syndrome (see Chapter 9) [29].
Dysarthria
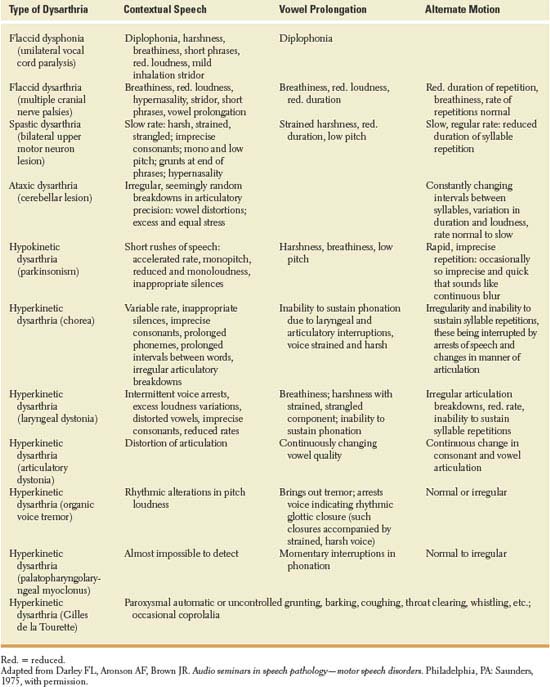
Dysarthria refers to impaired speech due to abnormal neuromuscular control and is manifest by abnormalities of articulation, respiration, prosody, resonance of voice, and phonation. Motor speech disorders may result from dysfunction at the upper motor neuron, lower motor neuron, cerebellar, extrapyramidal, or muscular level, with various characteristics of the dysarthria being of localizing value [9]. The evaluation of motor speech requires the assessment of three speech activities [9]:
1. A sample of contextual speech, including oral reading of a standard paragraph and evaluation of spontaneous speech
2. Vowel prolongation (ah …)
3. Alternate motion rate of the lips, tongue, and mandible (diadochokinesis), which is tested by having the patient repeat puh (labial), tuh (lingual), and kuh (guttural or posterior aspect of the tongue) rapidly and evenly
For example, an organic voice tremor (laryngeal tremor) may or may not be noted with contextual speech but may be brought out by vowel prolongation, during which oscillations of the voice are evident, at times resulting in speech arrest. Spastic dysarthria (from bilateral upper motor neuron lesions) has a harsh, strained, or strangled quality and slow rate during contextual speech, with slow, regular rate during alternate motion testing and strained harshness with low pitch in attempting vowel prolongation. When multiple levels of the neuraxis are affected, motor speech abnormalities are mixed (e.g., mixed flaccid and spastic dysarthria with amyotrophic lateral sclerosis; mixed spastic and ataxic dysarthria with multiple sclerosis; and mixed spastic, ataxic, and hypokinetic dysarthria with Wilson disease). Patients with progressive supranuclear palsy may have predominantly spastic, hypokinetic, and ataxic components, or a mixed dysarthria with a combination of spastic, hypokinetic, and ataxic components [19]. The various types of dysarthria and their characteristics are described in Table 14.1.
TABLE 14.1 Motor Speech Disorders