Chapter 13 Cerebellum
The cerebellum, the largest part of the hindbrain, is dorsal to the pons and medulla, and its median region is separated from them by the fourth ventricle. It is joined to the brain stem by three pairs of cerebellar peduncles, which contain afferent and efferent fibres. The cerebellum occupies the posterior cranial fossa, where it is covered by the tentorium cerebelli. It is roughly spherical but somewhat constricted in its median region and flattened; its greatest diameter is transverse. In adults, the weight ratio of cerebellum to cerebrum is approximately 1 : 10; in infants, it is approximately 1 : 20.
External Features and Relations
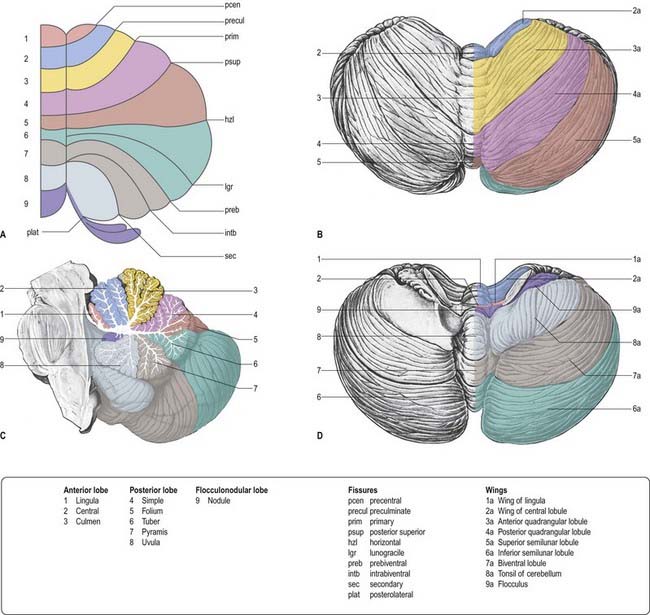
The cerebellum consists of two large, laterally located hemispheres that are united by a midline vermis (Figs 13.1–13.3). The superior surface of the cerebellum, which would constitute the anterior part of the unrolled cerebellar cortex, is relatively flat. The paramedian sulci are shallow, and the borders between vermis and hemispheres are indicated by kinks in the transverse fissures. The superior surface adjoins the tentorium cerebelli and projects beyond its free edge. The transverse sinus borders the cerebellum at the point where the superior and inferior surfaces meet. The inferior surface is characterized by a massive enlargement of the cerebellar hemispheres, which extends medially to overlie some of the vermis. Deep paramedian sulci demarcate the vermis from the hemispheres. Posteriorly, the hemispheres are separated by a deep vallecula, which contains the dural falx cerebelli. The inferior cerebellar surface lies against the occipital squama. The shape of the surface facing the brain stem is irregular. It forms the roof of the fourth ventricle and the lateral recesses on each side of it, while the cerebellar peduncles define the diamond shape of the ventricle when viewed from behind. Anterolaterally, the cerebellum lies against the posterior surface of the petrous part of the temporal bone.
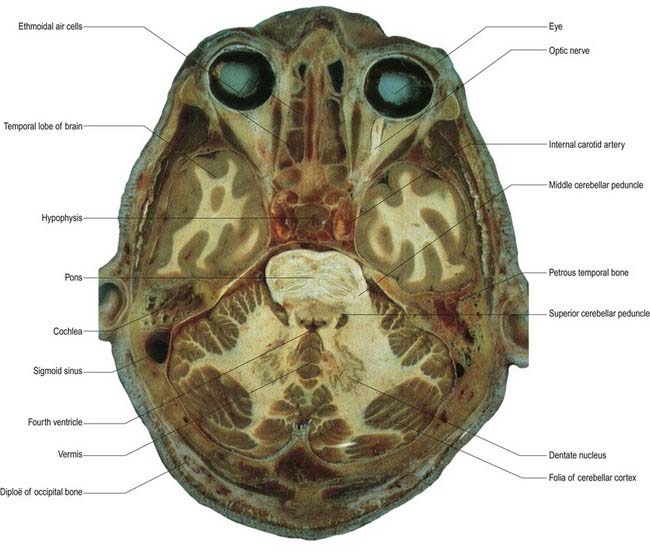
Fig. 13.1 Horizontal section through the cerebellum and brain stem.
(Courtesy of Dr. G. J. A. Maart.)
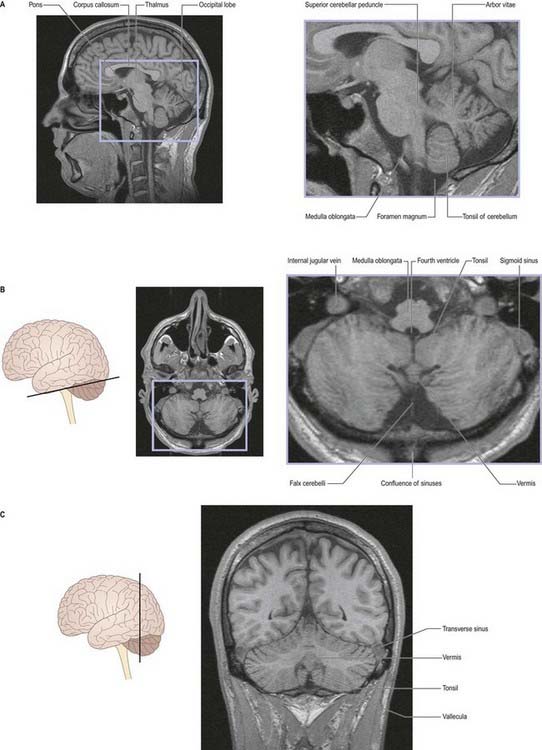
Fig. 13.2 Magnetic resonance images of the cerebellum of a 16-year-old girl. A, Sagittal slice. B, Coronal slice. C, Axial slice.
(Courtesy of Drs. J. P. Finn and T. Parrish, Northwestern University School of Medicine, Chicago, toddp@northwestern.edu.)
From the back forward, the inferior vermis is divided into the tuber, pyramis, uvula and nodule, in that order (see Fig. 13.3C). The tuber is continuous laterally with the inferior semilunar lobules and separated from the pyramis by the lunogracile fissure. The pyramis and attached biventral lobules (containing an intrabiventral fissure) are separated from the uvula and attached cerebellar tonsils by the secondary fissure. Behind the uvula, and separated from it by the median part of the posterolateral fissure, is the nodule. The tonsils are roughly spherical and overhang the foramen magnum on each side of the medulla oblongata.
Functional Divisions
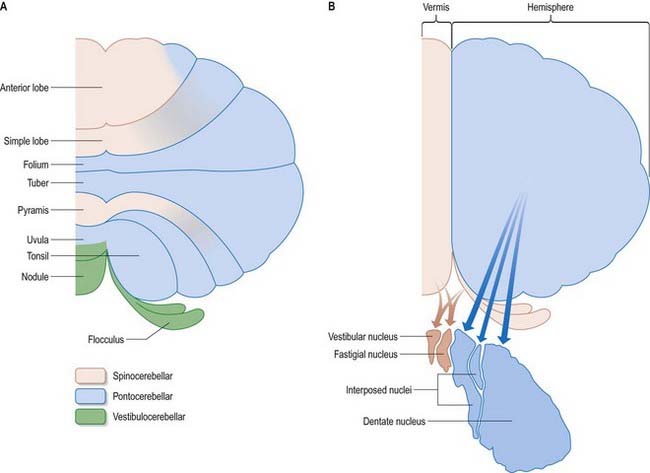
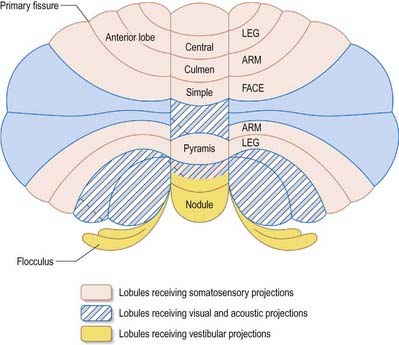
The cerebellum is divided functionally into a body, with inputs mainly from the spinal cord and pontine nuclei, and a flocculonodular lobe, which has strong afferent and efferent connections with the vestibular nuclei (Fig. 13.4). The body is subdivided into a series of regions dominated by their spinal or pontine inputs. The anterior lobe, simple lobule, pyramis and biventral lobules are the main recipients of spinal and trigeminal cerebellar afferents. Pontocerebellar input dominates in the folium, tuber and uvula and in the entire hemisphere, including those regions that receive afferents from the spinal cord.
Cerebellar Peduncles
Three peduncles connect the cerebellum with the rest of the brain (Figs 13.5, 13.6). The middle cerebellar peduncle is the most lateral and by far the largest of the three. It passes obliquely from the basal pons to the cerebellum and is composed almost entirely of fibres arising from the contralateral basal pontine nuclei, with a small addition from nuclei in the pontine tegmentum. The inferior cerebellar peduncle is located medial to the middle peduncle. It consists of an outer, compact fibre tract, the restiform (Latin for ‘rope-like’) body and a medial, juxtarestiform body. The restiform body is a purely afferent system. It receives the posterior spinocerebellar tract from the spinal cord and the trigeminocerebellar, cuneocerebellar, reticulocerebellar and olivocerebellar tracts from the medulla oblongata. The juxtarestiform body is mainly an efferent system. Apart from primary afferent fibres of the vestibular nerve and secondary afferent fibres from the vestibular nuclei, it is made up almost entirely of efferent Purkinje cell axons from the vestibulocerebellum, on their way to the vestibular nuclei, and the uncrossed fibres from the fastigial nucleus. The crossed fibres from the fastigial nucleus, after passing dorsal to the superior cerebellar peduncle, enter the brain stem as the uncinate fasciculus at the border of the juxtarestiform and restiform bodies.
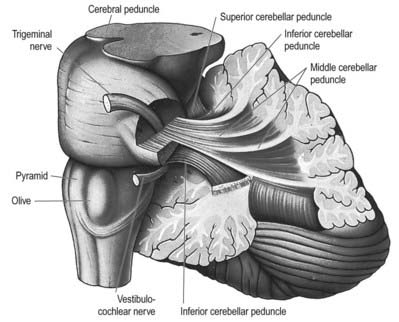
Fig. 13.5 Dissection of the left cerebellar hemisphere and its peduncles.
(Courtesy of Dr. E. B. Jamieson, University of Edinburgh.)

Fig. 13.6 Diagram illustrating the composition of the cerebellar peduncles. A, Dorsal view. B, Lateral view.
Internal Structure
Laterally, the medullary laminae merge into a large, central white mass that contains the four cerebellar nuclei: the dentate and the anterior (emboliform) and posterior (globose) interposed and fastigial nuclei (see Fig. 13.4). The dentate nucleus is the most lateral and largest and is an irregularly folded sheet of neurones that encloses a mass of fibres derived mainly from dentate neurones. It resembles a leather purse, the opening of which is directed medially. Fibres stream out through this so-called hilum to form the bulk of the superior cerebellar peduncle. The anterior and posterior interposed and fastigial nuclei lie medial to the dentate nucleus. The anterior interposed nucleus is continuous laterally with the dentate. The posterior interposed nucleus is medial to the anterior nucleus and is continuous with the fastigial nucleus, which is located next to the midline, bordering the fastigium (roof) of the fourth ventricle. Efferent fibres from the interposed nuclei join the superior cerebellar peduncle. A large proportion of the efferent fibres from the fastigial nucleus cross within the cerebellar white matter of the cerebellar commissure. After their decussation, they constitute the uncinate fasciculus (hook bundle), which passes dorsal to the superior cerebellar peduncle to enter the vestibular nuclei of the opposite side (see Fig. 13.6). Uncrossed fastigiobulbar fibres enter the vestibular nuclei by passing along the lateral angle of the fourth ventricle. Some fibres of the fastigial nucleus ascend in the superior cerebellar peduncle.
Cerebellar Cortex
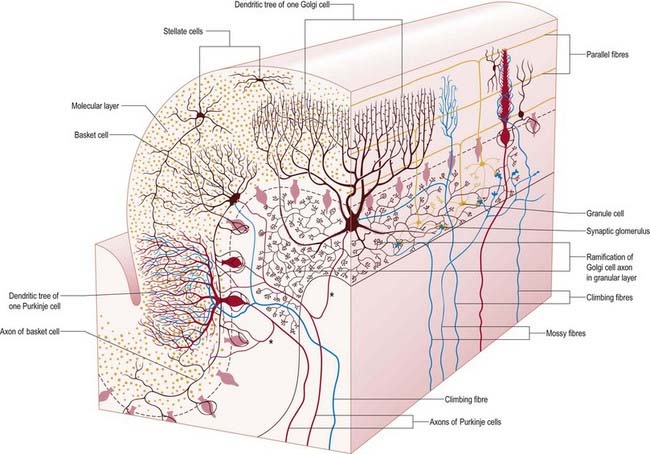
There are three main layers: molecular, Purkinje cell and granular (Fig. 13.7). The main circuit of the cerebellum involves granule cells, Purkinje cells and neurones in the cerebellar nuclei. Granule cells receive the terminals of the mossy fibre afferents (i.e. all afferent systems except the olivocerebellar fibres). The axons of the granule cells ascend to the molecular layer, where they bifurcate into parallel fibres (so called because they are oriented parallel to the transverse fissures and perpendicular to the dendritic trees of the Purkinje cells on which they terminate). Purkinje neurones are large and are the sole output cells of the cerebellar cortex. Their axons terminate in the cerebellar nuclei and vestibular nuclei. In addition to the dense array of parallel fibres, the dendritic trees of Purkinje cells receive terminals from climbing fibres whose neurones of origin are in the inferior olivary nucleus. The cerebellar cortex thus receives two distinct types of input: olivocerebellar climbing fibres, which synapse directly on Purkinje neurones, and mossy fibres, which connect to the Purkinje cells via granular neurones whose axons are the parallel fibres.
The granular layer (see Fig. 13.7) is approximately 100 µm thick in the fissures and 400 to 500 µm thick on foliar summits. There are approximately 2.7 million granular neurones per cubic millimetre. It has been estimated that the human cerebellum contains a total of 4.6 × 1010 granule cells and that there are 3000 granule cells for each Purkinje cell.
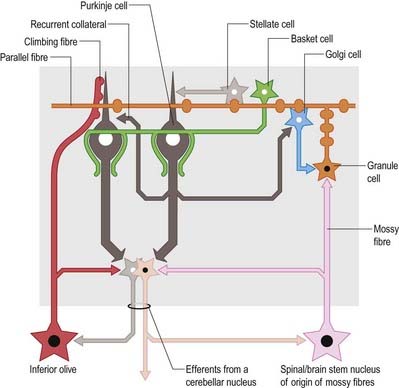
Of the five cell types described, the first four are inhibitory, liberating γ-aminobutyric acid (GABA), and the fifth is excitatory, liberating L-glutamate. Figure 13.8 summarizes their main connections.
Purkinje cells have a specific geometry that is conserved in all vertebrate classes (see Fig. 13.7). They are arranged in a single layer between the molecular and granular layers. Individual Purkinje cells are separated by approximately 50 µm transversely and 50 to 100 µm longitudinally. Their somata measure 50 to 70 µm vertically and 30 to 35 µm transversely. The subcellular structure of the Purkinje cell is similar to that of other neurones. One distinguishing feature is subsurface cisterns, often associated with mitochondria, that are present below the plasmalemma of somata and dendrites and may penetrate into the spines. They are intracellular calcium stores, which are important links in the second messenger systems of the cell.
The connections of the cerebellum are organized in two perpendicular planes, corresponding to the planar organization of the cerebellar cortex. Efferent connections of the cortex are disposed in parasagittal sheets or bundles that connect longitudinal strips of Purkinje cells with specific cerebellar or vestibular nuclei. The climbing fibre afferents to a Purkinje cell zone from the inferior olive display a similar zonal disposition. Cerebellar output is organized in modules, with a module consisting of one or more Purkinje cell zones, their cerebellar or vestibular target nucleus and their olivocerebellar climbing fibre input. Modular function is determined by the brain stem projections of the cerebellar or vestibular target nucleus. A general feature of the modular organization of the cerebellum is that GABAergic neurones in the cerebellar nuclei project to the subnuclei of the contralateral inferior olive, which give rise to their respective climbing fibre afferents. These recurrent connections are known as nucleo-olivary pathways.
Structural and Functional Cerebellar Localization
A double, mirrored localization exists in the anterior and posterior cerebellum (Fig. 13.9). The anterior lobe, simple lobule, pyramis and adjoining lobules of the hemisphere of the posterior lobe all receive branches from the same mossy and climbing fibres and project to the same cerebellar nuclei. The efferent pathways of these regions monitor the activity in the corticospinal tract and in the subcortical motor systems descending from the vestibular nuclei and reticular formation. The inputs to the cerebellum and the outputs from it are organized according to the same somatotopic patterns, but the orientation of these patterns is reversed. The representation of the head is found principally in the simple lobule and caudally in a corresponding region of the posterior lobe. The double representation of the body follows in rough somatotopic order. Vestibular connections of the cerebellum display a similar double representation in the most rostral lobules of the anterior lobe and far caudally in the vestibulocerebellum (Fig. 13.10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree