Examples of Doppler spectrum patterns observable with increasing ICP compatible with angiographic cessation of cerebral blood flow.
Imaging tips
Ultrasound systems and settings
All of the above-mentioned patterns can be depicted by ultrasound, regardless of whether Doppler or duplex ultrasound systems are being used. To achieve a detection of “low-flow” patterns, Doppler and duplex settings should be optimized accordingly. For Doppler spectrum analysis these comprise:
reduction of pulse repetition frequency
deactivation of filters
enlargement of Doppler sample width (10–15 mm)
increase of Doppler power and Doppler gain.
Duplex ultrasound systems allow additional optimization of the color-window settings, such as:
reduction of color-window size
reduction of color-window pulse repetition frequency
increase of color gain.
Steps of investigation
The following recommendations are derived from the Task Force Group on Cerebral Death of the Neurosonology Research Group of the World Federation of Neurology [18]. However, these might not be legally applicable or might be modified in different countries.
1. Before ultrasound analysis is started, establish cause of the coma, exclude reversible causes of coma (see “Introduction”), perform clinical investigation by two experienced examiners demonstrating no evidence of cerebral and brainstem function.
Comment: this usually includes testing for apnea.
2. Cerebral circulatory arrest can be diagnosed if the following Doppler spectrum patterns can be detected bilaterally, extra- and intracranially on two examinations at an interval of at least 30 minutes: alternating flow or systolic spikes (detailed characteristics mentioned earlier) in the intracranial ICA and MCA, respectively any branch or artery which can be recorded (anterior and posterior circulation, i.e., including the basilar artery via the transforaminal insonation approach). Intracranial findings must be confirmed by extracranial bilateral recording of the CCA, ICA and VA.
Comment: repetitive confirmative measurement with a time difference of at least 30 minutes is important, as very short time periods of reversed diastolic cerebral blood flow might not lead to brain death. Chiu and co-workers reported two infants with reversal of diastolic flow, one patient with status epilepticus and one patient with acute decompensating intracranial tumor. Acute treatment for increased ICP and status in the former and emergency tumor removal in the latter led to survival of both patients [19]. This is well in line with experiences from cardiac arrest, showing that irreversible loss of total brain function requires total cerebral ischemia periods of 10–15 minutes [18].
Requirements concerning the vessel segments to be documented vary greatly between countries. Some have adapted the above-mentioned criteria, in others, intracranial documentation of MCA and BA is sufficient. Most of the current guidelines do not require CCA insonation. This seems sensible, as this vessel also provides blood supply to the undisturbed external carotid artery which will often lead to a subsequent pulsatile but orthograde CCA flow.
The inclusion of the extracranial and intracranial ICA – although widely found within current guidelines – requires some additional consideration as the cessation of blood flow can be observed at different levels. An ICA flow cessation directly at the vessel offspring will produce the classical patterns of circulatory arrest. However, cases with supraophthalmic ICA flow cessation, that is, with a remaining flow via the ophthalmic artery into the eye, will not fulfill the above-mentioned criteria.
Instead, the extracranial ICA may have a detectable small orthograde flow. If analyzed via the transtemporal bone window in the pontine insonation planes or via the transorbital window the intracranial ophthalmic artery and the carotid siphon may also show a waveform with preserved orthograde diastolic flow component. The occurrence of this ICA flow pattern in patients in whom all other intracranial vessels show flow cessation has been reported using DSA and ultrasound [20–22]. The highest percent of up to 22% was described using transcranial Doppler via the transorbital approach in which ophthalmic artery flow and carotid siphon are ideally depicted [20]. With the growing awareness concerning insonation planes and exact vessel segment location – facilitated by the use of transcranial duplex ultrasound – it has to be expected that this finding will more frequently be seen using the transtemporal approach. A consequence at first will be the acceptance of an increasing number of false-negative results in all countries where guidelines require intracranial ICA insonation. Contrary to the suggestion of de Freitas [20] who recommended avoiding insonating the ICA via the transorbital approach, future guidelines could be adapted to the improved diagnostic capabilities of ultrasound, for example, by accepting ICA flow toward the eye and still diagnosing cerebral circulatory arrest if all other intracranial vessels show flow cessation. Although not yet focused on in current publications, the inclusion of the extracranial VA may also lead to false-negative results in those cases where VA blood flow is diverted via collaterals into the extracranial vasculature of the neck. Similarly, an adaptation of guidelines toward insonation of the V4-VA segments could solve this issue.
3. A lack of signal in transcranial insonation cannot be regarded as a safe sign of circulatory arrest as it might be caused by a missing transcranial bone window. A disappearance of previously seen flow signals (provided that the same ultrasound system and settings are being used) in combination with typical extracranial signals can, however, be accepted.
Comment: the shortcoming of a missing transtemporal bone window might be compensated by using the transorbital insonation approach. However, this requires energy settings, higher than the normally accepted mechanical index (MI) for optic bulb ultrasound to obtain good insonation results [23,24]. A recent publication reports on promising results using ultrasound contrast agents to improve signal yield. In this study, 27% of 102 patients for brain death diagnosis did not have sufficient insonation conditions. An intravenously applied bolus of 2.5 ml of SonoVue® reduced this number to only 3% of remaining inconclusive TCD examinations [25]. The study did not comment on the type of waveforms being observed, however, the mechanism of signal enhancement must have been a slow antegrade movement of the contrast microbubbles, triggered by the “to-and-fro” movements of the blood column.
A precondition for the detection of the above-listed pathological Doppler spectrum patterns is an intact skull bone. Openings of the skull, for example, after decompressive craniectomy or ventricular drains, are likely to prevent a sufficient general ICP rise, resulting in detectable vessel segments with some degree of preserved flow.
This problem applies to any form of disturbed skull integrity, also including severe head trauma with concomitant skull fracture as well as the newborn or young infants in whom the fontanels are still open. In adults as well as in infants the phenomenon of a patient fulfilling all clinical criteria of brain death including the loss of electroencephalographic (EEG) brain activity but remaining orthograde blood flow into the brain has repeatedly been reported using TCD but can also be observed by angiography [26,27,28,29,30]. It is important to note that in these cases, persisting blood flow is a “false-negative” finding and does not contradict irreversible loss of total brain function. Levels of hypoxic tolerance in brain and vasculature are different, that is, a 15-minute period of total brain hypoxia will lead to brain death, but not to a direct disintegration of the intracranial vascular structures, resulting in the above phenomenon. Diagnosis of brain death in these cases should therefore either be performed on clinical grounds or aided by electrophysiological ancillary testing like the EEG recording.
Diagnostic sensitivity and specificity of transcranial ultrasound varies, according to the applied criteria. Reported values of sensitivity range from 75% to 95%, specificity from 98% to 100% [31–33]. Individual studies have postulated single cases of “false-positive” ultrasound findings. However, if analyzed in detail, none of these cases would have been decided positive, if the above-discussed current guidelines were applied. Shortcoming in these single cases comprise single-time ultrasound analysis under hypothermia [21], single-time measurement, missing documentation of the insonated vessels [17], single-time ultrasound analysis in a hemodynamically unstable patient [34], ultrasound measurement before clinical diagnosis, accepting “any” three positive vessels documented over 3 minutes as circulatory arrest [35] and single-time ultrasound measurement of only MCAs [36,37,38].
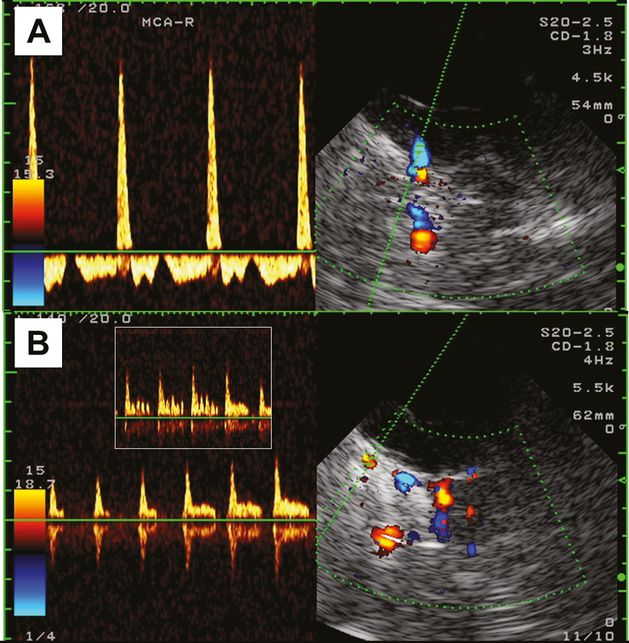
Example of a patient with typical alternating flow in all intracranially detectable arteries, i.e., MCA, ACA, PCA, V4-VA and BA. (A) Right MCA given as an example of the observed patterns. (B) Image and Doppler spectrum of the right intracranial ophthalmic artery (axial transtemporal insonation, upper pontine plane): Note the respiration-dependent partially preserved diastolic flow and a positive oscillation phenomenon during mild oscillation of the right optic bulb.
In conclusion, ultrasound assessment for detection of cerebral circulatory arrest is a noninvasive bedside technique with widespread availability and no reports of false-positive findings. The increasing use of transcranial duplex ultrasound will further facilitate confident vessel segment identification. A shortcoming of the technique is its restriction to patients with sufficient insonation conditions, that is, with patent transcranial bone windows. Its usefulness in patients with skull defects or in the very young with open fontanels is limited as these conditions lead to a high percentage of false-negative results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








