Cerebrovascular Diseases and EEG
Barbara Tettenborn
Ernst Niedermeyer
Donald L. Schomer
ROLE OF EEG IN DIAGNOSIS OF CEREBROVASCULAR DISEASE
Computed tomography (CT) and magnetic resonance imaging (MRI) are the major diagnostic tools in cerebrovascular disease considering the morphology is a vascular lesion. The major role of electroencephalography (EEG) is the evaluation of functional disturbances caused by cerebrovascular disease, mainly the detection of epileptogenic foci. Cerebrovascular events are acute, often catastrophic events that naturally have strong influences on the EEG. The tempo of evolution is an important factor. Acute vascular slow foci may be much more impressive than focal slowing caused by a slowly growing neoplasm. On the other hand, small deep vascular lesions are often too far distant from the cortex and may escape EEG detection, whereas deep vascular lesions in the brainstem and cerebellum reveal themselves only as secondary phenomena in the cerebral hemispheres. In an acute stroke, a massive and highly impressive EEG focus may be present before a CT scan can demonstrate the lesion (1). MRI has been found to be more precise and more informative than CT in the morphologic evaluation of stroke. The EEG is an indicator of abnormal physiologic function; the function of the involved central nervous system tissue breaks down before the structure shows its suffering. It has been shown experimentally (2) as well as clinically with the use of CT scan (3) that edema alone does not account for delta activity in EEG. In the modern view, the factor of ischemic tolerance of the brain has been critically analyzed (4) along with the demonstration of a large number of biomolecular substances presumed to be involved in this complex process.
The role of EEG as an indicator of disturbed function, locally as well as diffusely, in the event of a stroke has not always been duly appreciated. Decades ago, Hachinski and Norris (5) pointed out in their study on acute stroke that “electroencephalography is not much help in the diagnosis of stroke, especially when CT scanning is available.” But apposed to that, even in our days, including MRI techniques, it has to be stated that the evaluation of the degree and extension of cerebral dysfunction may be just as important as the demonstration of local or diffuse morphologic changes. An experienced electroencephalographer might be able to provide valuable information about the regional and general functional or dysfunctional state in a stroke. An imaginative approach to the EEG evaluation of strokes has been proposed by Velho-Groneberg (6). Accordingly, the EEG reflects dynamic processes that occur in the wake of a stroke.
In addition to local or more diffuse slow activity, the EEG also shows a more or less serious decline of physiologic patterns such as posterior alpha rhythm and intermixed slower or faster frequencies. Considerable localizing value has been ascribed to local depression of the background activity, whereas more extensive depression is regarded as somewhat less informative (7, 8 and 9). These authors believe that preservation of faster background activities, especially in subacute forms of middle cerebral artery (MCA) ischemia, is indicative of considerable neuronal survival in the infarcted zone and hence indicative of a good prognosis. Recent studies by Hilker et al. (10) could also indicate the absence of delta activity and presence of theta and fast beta frequencies within the EEG focus in stroke patients as predictor of a benign course, whereas diffuse slowing and focal slow delta frequency may point toward a more malignant course.
Even considering new MRI techniques, including functional MRI, EEG still has its place in diagnostic workup of stroke patients. Not only is MRI more expensive, but it also can be somewhat difficult to perform in the acutely ill stroke patient, whereas EEG can easily be done and repeated in practically every patient.
HYPERVENTILATION
Hyperventilation as a routine procedure in clinical EEG can activate otherwise latent EEG abnormalities in patients with cerebrovascular disorders. It is probably the hypoxic effect of hyperventilation that produces these changes. This activation can give rise to focal EEG slowing in cases of moyamoya disease (11). Kraaier et al. (12) investigated hyperventilation-induced changes with quantitative EEG and considered this method a model for transient ischemia in normal subjects.
RELEVANCE OF ACTIVATION METHODS TO THE ASSESSMENT OF CEREBROVASCULAR DISORDERS
At present, the role of these tests is rather limited. The fact remains, however, that these tests have given new insights into hemodynamic and neurophysiologic mechanisms underlying the EEG activity. An interesting overview of the cerebral hemodynamic evaluation has been presented by Carney et al. (13) with the EEG aspects of this work being aptly described by Anderson et al. (14).
ACUTE STROKE DETECTION
In the emergency evaluation of acute stroke, the progression from transient ischemia to infarction is a dynamic process, which is difficult to evaluate based solely on clinical grounds and anatomic imaging. Advances in CT and MRI scanning, including
diffusion-weighted MRI (DWI), are providing new insights into the pathophysiology of cerebral ischemia. Perfusion measures, such as perfusion-weighted imaging, may be able to estimate infarcted tissue with information about blood flow levels, but these measures may be less sensitive to dynamic changes in tissue viability than the EEG. Within seconds, the EEG reflects changes in cerebral blood flow and metabolism. Both human and animal evidence indicates that the ischemic cascade is rapid, with marked changes occurring over seconds and minutes, and irreversible changes occurring within a few hours. DWI and EEG detect pathophysiologic responses to reduced blood flow around the same range, between 30 and 40 mL/100 g/min (15), suggesting that they are comparable in their sensitivity to the stage of ischemic gradient. The accuracy of EEG scalp recordings, however, may be limited by adequate sampling of the electrical field. Biophysical simulations have indicated for many years that the spatial frequency of the scalp EEG requires higher sampling densities to avoid artificial smoothing of the scalp potential field caused by undersampling (16,17). Luu et al. (18), in their study on acute stroke patients, found within 8 to 36 hours after symptom onset using a 128-channel sensor montage, that an accurate description of stroke-related topographic EEG changes is dependent on adequate spatial sampling density. Accurate description of the spatial distribution of the stroke-related EEG was achieved only with the 64- and 128-channel EEG. As the recording density decreases to 32 channels, the distribution of the scalp EEG spectra is distorted, potentially resulting in mislocalization of the affected region. Their results demonstrated that the EEG acquired with the conventional 19-channel montage is clinically useful, if the data are reviewed by an expert, but localizing information is limited by the available data. Nevertheless, for daily clinical routine 128-channel EEG recording for every stroke patient is not realistic. Therefore, studies on the value of conventional 19-channel montage using the international 10-20 system are currently being done. Conventional EEG recordings could be useful in predicting the subgroup of acute stroke patients who are predisposed to develop epileptic seizures in the follow-up.
diffusion-weighted MRI (DWI), are providing new insights into the pathophysiology of cerebral ischemia. Perfusion measures, such as perfusion-weighted imaging, may be able to estimate infarcted tissue with information about blood flow levels, but these measures may be less sensitive to dynamic changes in tissue viability than the EEG. Within seconds, the EEG reflects changes in cerebral blood flow and metabolism. Both human and animal evidence indicates that the ischemic cascade is rapid, with marked changes occurring over seconds and minutes, and irreversible changes occurring within a few hours. DWI and EEG detect pathophysiologic responses to reduced blood flow around the same range, between 30 and 40 mL/100 g/min (15), suggesting that they are comparable in their sensitivity to the stage of ischemic gradient. The accuracy of EEG scalp recordings, however, may be limited by adequate sampling of the electrical field. Biophysical simulations have indicated for many years that the spatial frequency of the scalp EEG requires higher sampling densities to avoid artificial smoothing of the scalp potential field caused by undersampling (16,17). Luu et al. (18), in their study on acute stroke patients, found within 8 to 36 hours after symptom onset using a 128-channel sensor montage, that an accurate description of stroke-related topographic EEG changes is dependent on adequate spatial sampling density. Accurate description of the spatial distribution of the stroke-related EEG was achieved only with the 64- and 128-channel EEG. As the recording density decreases to 32 channels, the distribution of the scalp EEG spectra is distorted, potentially resulting in mislocalization of the affected region. Their results demonstrated that the EEG acquired with the conventional 19-channel montage is clinically useful, if the data are reviewed by an expert, but localizing information is limited by the available data. Nevertheless, for daily clinical routine 128-channel EEG recording for every stroke patient is not realistic. Therefore, studies on the value of conventional 19-channel montage using the international 10-20 system are currently being done. Conventional EEG recordings could be useful in predicting the subgroup of acute stroke patients who are predisposed to develop epileptic seizures in the follow-up.
In patients with large MCA infarction, space-occupying brain edema may lead to a malignant course with up to 80% mortality using conservative treatment. As interventional treatment strategies must be started before the deterioration occurs, predictors of a malignant course are necessary. Hilker et al. (10) investigated the value of EEG within 24 hours after the onset of stroke in 25 patients suffering a large MCA infarction. Their findings indicated that the absence of delta activity and the presence of theta and fast beta frequencies within the focus predict a benign course, whereas diffuse generalized slowing and slow delta activity in the ischemic hemisphere may point toward a malignant course. Therefore, EEG can deliver useful information to select those patients who develop malignant edema.
DETERIORATING STROKES
Various complications may interfere with recovery from a stroke. The term “deteriorating stroke” has been used by Hachinski and Norris (5) and includes progressively evolving strokes, recurrent strokes, and also spatially extending strokes. Their causes may be thrombotic, embolic, or hemorrhagic. Important pathogenic mechanisms include cerebral edema and the syndrome of inappropriate antidiuretic hormone, which leads to reduction of cerebral sodium, potassium, and chloride content, altered brain energy metabolism, and neurotransmitter amino acid uptake (5). Cardiac factors, poststroke depression, and drug effects also may lead to poststroke deterioration. In evolving and deteriorating strokes, the ischemic penumbra plays a major role (19). In deteriorating strokes, the EEG slowing tends to become more diffuse. It also may reflect drug effects (sedatives), but it is apt to remain notably unaltered in the case of poststroke depression.
EEG IN HEMISPHERIC STROKE
The most common cause of a hemispheric ischemic lesion is atherosclerotic disease of the internal carotid artery or MCA. Size of infarct and clinical symptoms relate to localization and to collateral circulation. An infarct may encompass the entire territory supplied by carotid artery, followed in a few days by severe edema, raised intracranial pressure, transtentorial herniation, and even death. At the other extreme, occlusion of carotid artery may occur without obvious clinical deficits, because of adequate collateral circulation. In acute stages, EEG is usually the most sensitive laboratory index. Massive hemispheric infarct due to carotid occlusion causes marked EEG abnormalities (20, 21 and 22):
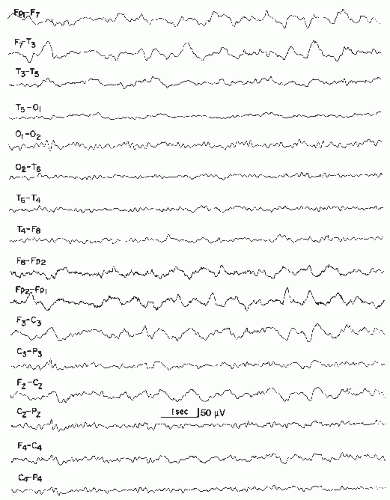
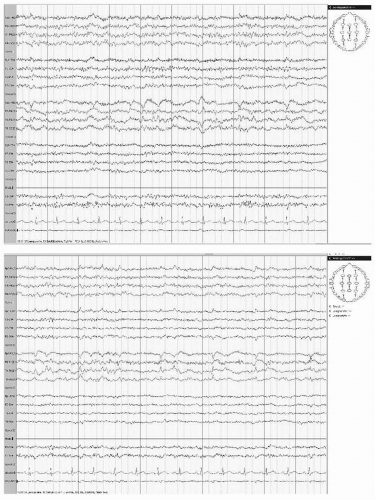
Widespread arrhythmic delta activity occurs over the involved hemisphere, most prominently in temporal or frontotemporal regions (Fig. 19.1). In patients with smaller hemispheric infarcts, EEG from uninvolved hemisphere usually remains normal.
Ipsilateral alpha rhythm may be absent or of lower amplitude and slower frequency. Reactivity of alpha rhythm may diminish or disappear (Fig. 19.2). Rolandic beta activity and sleep spindles may also disappear.
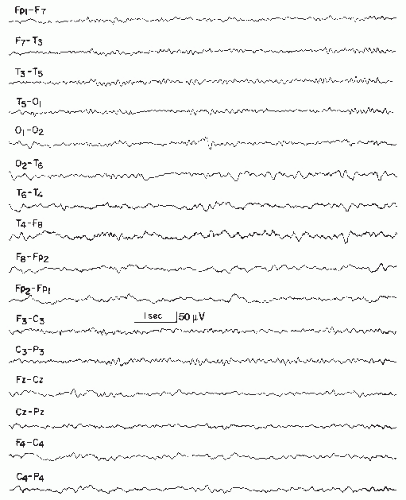
Extensive infarcts may markedly decrease or “suppress” electrocerebral activity, normal and abnormal, over the affected hemisphere during the first few days. In patients with smaller hemispheric infarcts, EEG from uninvolved hemisphere usually remains normal. With extensive infarcts causing cerebral edema and displacement of midline structures, variable degrees of slow activity appear in contralateral hemicranium, often with bilateral intermittent rhythmic 2 to 3 per second delta activity (IRDA) (Fig. 19.3), which may be marked in the frontal region (FIRDA, frontal intermittent rhythmic delta activity) (Fig. 19.4). In children, this rhythmic delta activity may be most marked occipitally (OIRDA, occipital intermittent rhythmic delta activity). Marked diffuse EEG changes may make lateralization difficult; also, massive infarcts causing ipsilateral voltage depression and prominent contralateral slow activity can lead to erroneous lateralization.
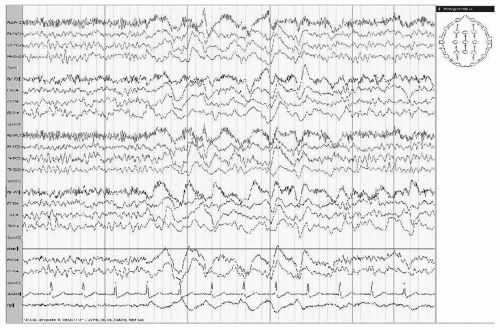
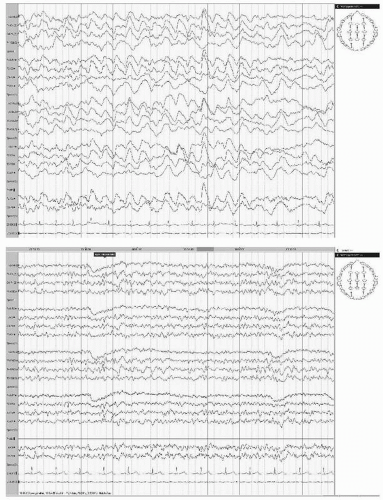
With acute hemispheric infarctions, particularly embolic, epileptiform activity occurs commonly and tends to be periodic (periodic lateralized epileptiform discharges [PLEDs]) (Fig. 19.5). Such patients are usually obtunded and have focal seizures with corresponding neurologic deficits. PLEDs and clinical manifestations usually resolve in 1 to 2 weeks.
During the first days after infarction, EEG abnormalities are most prominent, with extent and voltage of slow activity progressively declining. Few prospective studies have evaluated EEGs serially in a carefully defined population. Kayser-Gatchalian and Neuendörfer (20) did EEGs on 79 patients with infarcts in carotid distribution. Initial EEGs were done within 48 hours after infarction and again on days 5, 10, and 22. They visually classified EEG abnormalities into “background slowing” and “focal changes,” with each group being divided by defined criteria into the following categories: “none,” “mild,” “moderate,” or “severe.” In the initial EEGs, “background slowing” reliably predicted state of consciousness: only 1 of 14 patients with severe or moderate slowing was alert, whereas 33 of 44 patients without slowing were alert. Focal changes paralleled motor deficits: 39 of 51 patients with moderate or severe focal changes had severe paresis or hemiplegia, whereas only 7 of 17 patients without focal changes had severe weakness. Background slowing in the initial EEG predicted outcome on day 22: only 2 of 14 patients with moderate or severe slowing improved, whereas 38 of 44 patients without slowing improved. Persistent focal changes paralleled persistent severe hemiparesis or hemiplegia. In a smaller series of 15 patients, Sainio et al. (23) compared visual with spectral analysis. Results of visual analysis equaled spectral analysis, except for a lower incidence of focal abnormalities; in general, their findings paralleled the findings of Kayser-Gatchalian and Neuendörfer (20). Although unusual in the acute phase of the infarction, epileptiform discharges can evolve with time, possibly heralding postinfarction seizures.
In patients with large MCA infarction, space-occupying brain edema may lead to a malignant course with up to 80% mortality under conservative treatment. As interventional treatment strategies must be started before the deterioration occurs, predictors of a malignant course are necessary. Burghaus et al. (24) reported the results of a study on early EEG within 24 hours after the onset of stroke in 25 patients suffering a large MCA infarct (12 patients with a malignant and 13 with a nonmalignant course). EEG analysis was performed according to wellestablished indicators for focal as well as global changes. Their results indicated that the absence of delta activity and the presence of theta and fast beta frequencies within the focus predict a benign course (P < 0.05), whereas diffuse generalized slowing and slow delta activity in the ischemic hemisphere may point to a malignant course.
Infarction due to occlusion of the anterior cerebral artery is rare. EEG findings consist of (i) ipsilateral frontal delta activity (either arrhythmic or rhythmic, or both) and (ii) depression of anterior beta activity.
Occlusion of the posterior cerebral artery may result in (i) infarction of the mesial temporal lobe with arrhythmic delta activity over the posterior head region and (ii) marked disorganization or absence of alpha rhythm.
PLEDS: CLINICAL CORRELATES
PLEDs are a relatively uncommon electroencephalographic pattern characterized by lateralized or focal periodic or nearperiodic spike, spike-wave, or sharp wave complexes present throughout most or all of the recording (Fig. 19.5B). Chatrian et al. introduced the term PLEDs in 1964 (25), though the phenomenon was first described in 1952 by Echlin et al. (26). PLEDs occur in all age groups, from infants to adults. They are usually seen transiently in the setting of an acute destructive cerebral lesion and occur early in the course of patient illness. They can be seen less commonly with systemic disturbances and a remote cerebral lesion. Rare chronic PLEDs, persisting for a period of 3 months to more than 20 years, have been reported in patients with chronic brain lesions and associated partial seizure disorders. Bilateral, independently occurring PLEDs (BiPLEDs) were recognized by Chatrian in 1964 (25) and formally characterized in 1981 by de la Paz and Brenner (27). They are seen in the setting of multifocal or diffuse cerebral injury, such as anoxia, and herald a less favorable prognosis with higher mortality. Approximately 80% to 90% of patients with PLEDs experience clinical seizure activity, primarily focal motor seizures. In 1991, Reiher et al. (28) described the entity “PLEDs plus,” characterized by PLEDs admixed with high-frequency, low-voltage “polyspike” rhythms. These have an even stronger correlation with clinical seizures and status epilepticus. PLEDs are generally not considered an ictal pattern, though this has been reported, and remains a matter of ongoing debate (29, 30 and 31).
EEG IN VERTEBROBASILAR ISCHEMIC DISEASE
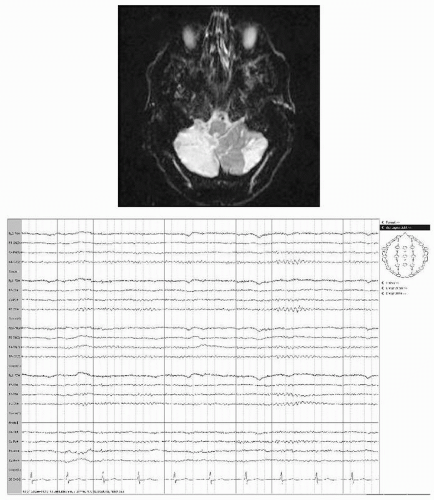
Ischemia in the vertebrobasilar territory includes infarction of brainstem, diencephalon, or cerebellum with varying neurologic deficits depending on specific artery involved and extent and location of the infarct. Disparity between extensive neurologic deficits and EEG changes characterize brainstem and also most cerebellar infarcts (Figs. 19.6 and 19.7). Less than one third of patients with limited infarcts to the brainstem show even slightly altered EEGs (32). Basilar artery occlusion infarcting ventral pons but sparing tegmentum causes a distinctive clinical picture
known as “deefferented” or “locked-in” syndrome (33). Patients are alert but are mute and tetraplegic, with the only preserved motor acts being blinks and vertical eye movements. In these patients, EEG is normal (34,35); the alpha rhythm reacts to various stimuli, including intermittent photic stimulation that produces photic responses. Patients with bilateral pontine infarcts involving tegmentum show striking dissociation between clinical and EEG findings (25,36). Despite coma and extensive bilateral motor deficits, EEGs may show activity of alpha frequency. However, this alpha activity fails to react to various stimuli such as intermittent photic stimulation; hence, it is abnormal. Because of the association of coma and alpha activity, the term “alpha coma” has been applied to such patients (37). A similar association also occurs in patients rendered comatose by cardiopulmonary arrest (38) or drug overdose (39).
known as “deefferented” or “locked-in” syndrome (33). Patients are alert but are mute and tetraplegic, with the only preserved motor acts being blinks and vertical eye movements. In these patients, EEG is normal (34,35); the alpha rhythm reacts to various stimuli, including intermittent photic stimulation that produces photic responses. Patients with bilateral pontine infarcts involving tegmentum show striking dissociation between clinical and EEG findings (25,36). Despite coma and extensive bilateral motor deficits, EEGs may show activity of alpha frequency. However, this alpha activity fails to react to various stimuli such as intermittent photic stimulation; hence, it is abnormal. Because of the association of coma and alpha activity, the term “alpha coma” has been applied to such patients (37). A similar association also occurs in patients rendered comatose by cardiopulmonary arrest (38) or drug overdose (39).
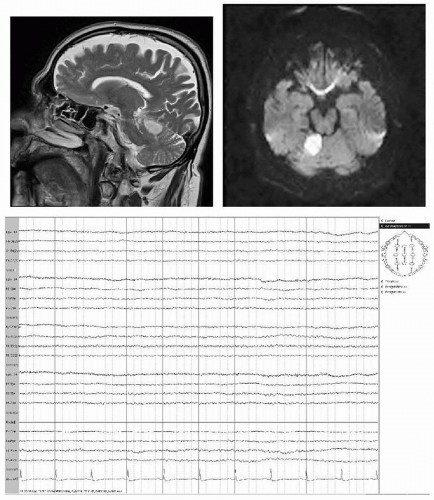 Figure 19.7 A 69-year-old male with sudden onset of nausea, dizziness, and weakness of right arm. MRI shows infarction in the territory of the superior cerebellar artery. EEG is normal. |
At more rostral level, infarcts of midbrain and diencephalon cause not only coma but also EEG alterations. Bilaterally synchronous and symmetrical theta-delta activity, sometimes maximal over frontal regions, replaces normal rhythms. Nevertheless, these relatively mild changes, compared with the depth of coma and extent of neurologic deficits, should alert EEGers to an intrinsic brainstem lesion. Thrombosis of rostral portion of basilar artery can also cause infarcts of occipital lobes. EEGs show posterior arrhythmic delta activity with loss of alpha rhythm, unilaterally or bilaterally.
Jouvet (40) has correlated sites of brainstem lesions with alterations in consciousness and changes in EEG activity. He has concluded the following:
Lesions of medulla oblongata and caudal pons alter usually neither consciousness nor EEG.
Extensive lesions of upper pons and lower mesencephalon involving more than one half of tegmentum usually cause coma. EEGs may show alpha activity that does not react to external stimuli. Although vital in maintaining consciousness, mesopontine regions seemingly contribute little to genesis of EEG activity.
Lesions of rostral brainstem and caudal diencephalon cause not only deep coma but also diffuse, nonreactive theta and delta activity.
In bilateral paramedian thalamic infarction, triphasic waves can appear on electroencephalogram at acute stage (41). Triphasic waves are nonspecific EEG findings occurring in both metabolic and nonmetabolic conditions. The triphasic waves in bilateral paramedian thalamic infarction might be related to level of consciousness and not necessarily predictive of poor prognosis.
EEG IN SUBCORTICAL ARTERIOSCLEROTIC ENCEPHALOPATHY
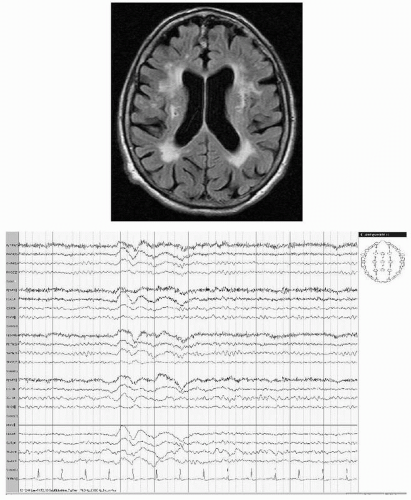
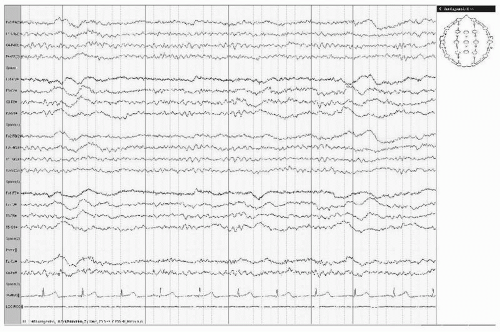
The incidence of epileptic seizures in subcortical vascular encephalopathy is more related to a higher rate of lacunae within the subcortical white matter rather than in the basal ganglia and brainstem. The higher incidence of epilepsy in this group can be explained by diaschisis resulting in suppression of the inhibitory cortical activity (42). Positron emission tomographic examination of the brain has confirmed that patients with leukoaraiosis and seizures have a more severe decline of regional cerebral blood flow and metabolic rate of oxygen in the whole cortex compared to those without seizures (43) and to those with late-onset symptomatic seizures (44). Patients with extended chronic subcortical infarctions can show generalized intermittent delta activity on EEG recording (Fig. 19.8).
EEG IN CEREBRAL ISCHEMIC DISEASE DUE TO EMBOLISM
All forms of cerebral emboli can cause a variety of clinical and EEG changes. Already decades ago, Lhermitte and his coworkers demonstrated that a surprisingly large number of strokes believed to be thrombotic are due to emboli of cardiac origin (45, 46 and 47). Because of new imaging techniques and more insight into pathophysiology of cerebral ischemia, it is well known that about 40% of all cerebral ischemic events are embolic in origin. Emboli can either arise from thrombotic material within the larger brain supplying arteries (common carotid or internal carotid arteries, vertebral arteries) or they can be cardiac in origin. Aortic arch plaque material may also cause cerebral ischemia. Embolism of thrombotic material and gas and fat emboli may give rise to clinically and electroencephalographically similar pictures (Fig. 19.9A,B). In embolism, however, the extremely acute shock-like effect may trigger a chain of vasomotor responses in adjacent vascular territories. These responses either hasten the catastrophic events or resolve quickly so that recovery may evolve with unexpected speed.
Rasheva (48) and Rasheva et al. (49) noted clinical epileptic seizures in 10.1% and paroxysmal EEG discharges (spikes and sharp waves) in 57.1% of a large population of 328 patients with embolic stroke.
The importance of cerebral gas embolism during and after cardiac surgery is widely known. According to Naquet and Vigouroux (50), gas emboli may produce EEG changes, similar to those seen in watershed infarction, to be discussed in the following section. In fat embolism, Müller and Klingler (51) reported marked delta-theta activity of frontotemporal accentuation. Similar findings were reported by Scherzer (52).
Experimental cerebral embolism lends itself to the study of immediate EEG effects. These are dramatic and instantaneous. In the rabbit, Hegedüs et al. (53) demonstrated abrupt flattening of the affected hemisphere after embolization of the MCA; there was even some voltage decrease contralaterally (Fig. 19.3). This clearly shows that the effects of a major embolism extend beyond the involved arterial territory. But in general, the EEG cannot effectively assist in the differentiation of thrombotic and embolic cerebral ischemia.
WATERSHED ISCHEMIA
Ischemic states usually pertain to the territory of the involved artery, but another mechanism has become clinically important. This is the concept of “extraterritorial ischemia” that gives rise to “watershed infarctions” (“boundary infarctions”) along the borders of the major arterial territories, between middle,
posterior, and anterior cerebral arteries. This mechanism is based on a decline in the systemic circulation. This is a drop in blood pressure (BP) or cardiac output, usually combined with a diseased and plaque-ridden cerebral vasculature. It is a logical and convincing concept when it is assumed that boundary areas between the major cerebral arterial territories are most likely to suffer from ischemia. The work of Zülch and Behrend (54) clearly demonstrates the distinction between the watershed type and the classical obstructive ischemic infarction.
posterior, and anterior cerebral arteries. This mechanism is based on a decline in the systemic circulation. This is a drop in blood pressure (BP) or cardiac output, usually combined with a diseased and plaque-ridden cerebral vasculature. It is a logical and convincing concept when it is assumed that boundary areas between the major cerebral arterial territories are most likely to suffer from ischemia. The work of Zülch and Behrend (54) clearly demonstrates the distinction between the watershed type and the classical obstructive ischemic infarction.
Acute watershed-type ischemia is most commonly found in elderly patients with a history of cerebral arteriosclerosis and chronic cardiovascular problems. A variety of illnesses may trigger the acute cerebral ischemia. Acute infection, dehydration, uncontrolled diabetes mellitus, cardiovascular decompensation, severe anemia, recent craniocerebral trauma, extracerebral embolism, liver disease with portocaval encephalopathy, and severe arterial hypertension are events that can facilitate the occurrence of watershed ischemia (50). Clinically, a contralateral hemiparetic deficit is usually present, but it may not be as pronounced as in primary capsular ischemia or hemorrhage.
The EEG is slow with a mixed delta-theta range frequency and severely disorganized. These changes are essentially diffuse with consistent lateralization to the affected hemisphere. Against this slow and disorganized background of rather moderate voltage, there are consistently repetitive large sharp waves or spikes, often compounded and consisting of several subcomponents and phases, usually over the posterotemporal-occipital-parietal region of the hemisphere that harbors the lesion. They are called PLEDS. This periodic spike discharge is rhythmically or quasirhythmically repetitive at a rate of approximately 1 per second. The discharge may become quite widespread, even reaching into the other hemisphere. Occasionally, the periodic spikes show a superior frontal maximum.
At this stage of repetitive spike activity, contralateral focal motor twitching (fingers or hand, face, abdomen, leg, foot, or toes) is the common accompaniment of the periodic spikes, but such PLEDs often occur without accompanying motor activity. Further work on these discharges was presented by Alajouanine et al. (55), Barolin et al. (56), Chatrian et al. (25), Markand and Daly (57), and Pohlmann-Eden et al. (29,30). These latter authors feel that PLEDs indicate focal hyperexcitability in the penumbra zone of an ischemic stroke. Multifocal epileptic seizures of partial character may also evolve in watershed ischemia, along with independent EEG foci (58).
EEG IN TRANSIENT ISCHEMIC ATTACKS
Transient ischemic attacks (TIAs) are defined as a temporary, clinically completely reversible event of focal, nonconvulsive, neurologic dysfunction secondary to a failure of sufficient blood supply to a distinct area of the brain, retina, or other nervous tissue. Most TIAs last less than 15 minutes, but the old purely clinical standard definition of TIA was that symptoms and signs have to be resolved within 24 hours (59,60). According to this definition DWI demonstrated acute permanent ischemic lesions in approximately 30% of TIA patients, climbing to 50% when DWI is combined with perfusion-weighted imaging (61). A new proposed definition by the TIA Working Group is that TIA is a brief episode of neurologic dysfunction caused by focal brain or retinal ischemia with clinical symptoms typically lasting less than 1 hour, most often some minutes, without evidence of acute infarction on modern neuroimaging (61,62).
In some patients, TIA presents with the symptomatology of limb shaking with brief, arrhythmic flailing or jerking movements of the arm or leg, which can be easily misdiagnosed as a seizure disorder. But early diagnosis of a cerebrovascular disorder is important for immediate initiation of appropriate secondary stroke prevention including medical and interventional or surgical procedures. EEG testing is often ordered in a patient with limb shaking TIA because of suspicion of seizures. Taghavy and Hamer (60) reviewed the records of patients with TIAs over a period of 6 years. In 79 patients with clinical TIA, an EEG was performed, in 44.3% demonstrating focal abnormal electrical activity after recovery from TIA corresponding to the affected hemisphere. In only 17.7% of these patients, there was also an appropriate hypodensity in cranial CT scan. Failure to consider cerebral hypoperfusion as a potential cause of focal delta EEG slowing in the presence of normal structural imaging studies may contribute to delay in correct diagnosis.
The above mentioned new TIA definition makes it even more difficult to distinguish TIAs from seizures, especially from inhibitory seizures in elderly patients (63). Recognition of modern diagnostic clues can prompt expedited magnetic resonance angiography (MRA), carotid duplex ultrasound, or computed tomography angiography (CTA) studies of the neck and intracranial blood vessels to identify arterial stenoses or occlusions (64).
De Reuck and Van Maele (63) performed a study on TIAs and inhibitory seizures in elderly patients and included 25 patients with inhibitory seizures and 252 patients with TIA over a 7-year study period taking into account the old and new TIA definition. The clinical characteristics and the EEG findings were compared. Temporary speech disturbance, associated with some partial amnesia for the event, was the most common clinical presentation of an inhibitory seizure. Additionally, specific and nonspecific postictal EEG abnormalities were observed in the majority of inhibitory seizure patients, whereas the EEG was normal in more than 90% of TIA patients. Diffuse slowing and intermittent rhythmic delta activities were only observed in patients with seizures, not in TIA patients. They concluded that it should always be checked whether the patient is able to recall the episode of focal disturbance completely, as more than two thirds of patients with an inhibitory seizure will have difficulties to describe their temporary deficit clearly.
EEG IN INTRACRANIAL VENOUS THROMBOSIS
In the era of antibiotic therapy, episodes of infectious intracranial venous thrombosis and thrombophlebitis have become rare. Complicated ear infections (transverse sinus) or facial carbuncles (cavernous sinus) occasionally cause such a progression of infectious material into the venous system of the cranial cavity. Complications of abortion are another cause; widespread cortical venous thrombosis may develop intrapartum. Reviews of the field have been presented by Garcin and Pestel (67), Dubois (68), Huhn (69,70), and Carter (71).
EEG changes in cortical venous thrombosis intrapartum can be very pronounced, with widespread prominent delta activity; these patients are not necessarily in a state of impaired consciousness but show marked changes of higher cortical functions, such as aphasia, mutism, or apraxia, and the course of ensuing recovery may be very slow.
Literature on the EEG in cerebrovenous pathology is scanty. The work of Huhn (69), Lemmi and Little (72), Franco (73), and Hubach and Struck (74) has been particularly informative. Van der Drift and Kok (9) demonstrated the underlying pathophysiologic mechanisms as well as the correlations of EEG and venous pathology. Focal slowing and ictal epileptic activity have been reported (75).
ROLE OF EEG IN PROGNOSIS OF EPILEPTIC SEIZURES AFTER ISCHEMIC STROKE
The incidence rates for epilepsies have two peaks regarding age: one in the first decade of life and a second at higher age with a steep increase between age 70 and 80 (76). Cerebral ischemia is the single most common cause of first seizures and epilepsy in later life above the age of 60 years, ahead of degenerative disorders, brain tumors and head trauma, accounting for up to one third of newly diagnosed seizures in this population. The leading type of stroke-related seizures is focal with a high rate of secondary generalization (77, 78, 79, 80, 81, 82, 83, 84, 85 and 86).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree